About Compartmental Syndromes
What is commpartmental syndrome?
A compartmental syndrome is a condition in which increased pressure within a limited space compromises the circulation and function of the tissues within that space. This condition is a cause of major loss of function, limb, and even life. It can result from trauma, prolonged recumbancy (in surgery or resulting from drugs or alcohol), or physical activity. It is common enough to affect thousands of individuals each year yet rare enough that each physician may encounter it only once or twice during his or her career.
Summary
Compartmental syndromes challenge even the best clinicians. These syndromes occur when locally increased tissue pressure compromises local circulation and neuromuscular function. The incidence of compartmental syndromes is rising along with the frequency of their various etiologies: extremity trauma, limb ischemia, intensive use of muscles, extremity surgery, and drug and alcohol abuse. Despite this increase in frequency, the compartmental syndrome remains sufficiently uncommon in the experience of the average practitioner that he may be unfamiliar with its diagnosis and management. Because prompt treatment of compartmental syndromes is essential, the consequences of this unfamiliarity may be serious. Even to those most familiar with them, compartmental syndromes pose major problems in pathogenesis, diagnosis, and treatment. For example, the precise effect of increased tissue pressure on the microcirculation-the key to understanding compartmental syndromes-remains a matter of considerable conjecture.
The clinical diagnosis of a compartmental syndrome is frequently made difficult by the fact that other conditions may produce similar symptoms and signs. Although new diagnostic methods, such as tissue pressure measurement, have been described, they have failed to completely resolve these problems of differential diagnosis. This failure is a result of practical problems in the application of these techniques and in the interpretation of their results. Thus, the physician is called upon to synthesize all the available information in arriving at the correct diagnosis.
Adequate treatment of compartmental syndromes requires the wide opening of all potentially affected compartments. Unfortunately the institution of this treatment is often delayed in the hope that the compartmental syndrome will resolve spontaneously. Even if prompt surgery is performed the functional result may be compromised by an incomplete decompression carried out in the hope of a superior cosmetic result. Special problems may be presented by the surgical wound after decompression and by fractures that are associated with compartmental syndromes. Compartmental syndromes may give rise to significant complications that include infection and myoglobinuric renal failure.
Definition of the compartmental syndrome
There is a vital need for new organization of the literature on compartmental syndromes. Attempts to locate the relevant articles are frustrated by the lack of an appropriate indexing system. For example, the Index Medicus has entries only for "Volkmann's ischemia" and "anterior compartment syndrome"; thus it is difficult to know where to locate information on compartmental syndromes in other locations. Furthermore, in the Journal of Bone and Joint Surgery Quinquennial Index (1973-1977) all compartmental syndromes are listed under "Volkmann's ischemia." Finally, Sheridan and Matsen's classic article "Fasciotomy in the treatment of the acute compartment syndrome"1 is listed under the following headings in the Medline system: acute disease adolescents adults aged child fascia/ surgery female human male middle age neuromuscular disease/ surgery postoperative complications/etiology syndrome and time factors. Thus this important article could not be located by a search that requests articles dealing with compartmental syndromes, tissue pressure, or even ischemia.
A further example of the confusion resulting from the lack of organized nomenclature may be found in the 1979 American Academy of Orthopedic Surgeons Orthopedic In-Training Examination (page 5 question T3). The question concerns the early signs of "impending Volkmann's ischemic contracture and for the answer we are referred to an article on anterior tibial compartment syndrome." These two terms bear little apparent relation to one another.
Use of the literature is confused even further by a plethora of other names used to refer to the compromise of local circulation by increased tissue pressure.
Being aware of the problems with the existing nomenclature I proposed a system for referring to those conditions in which pressure-induced circulatory compromise plays a central role.2l This proposed nomenclature is based on the following definition: A compartmental syndrome is a condition in which increased pressure within a limited space compromises the circulation and function of the tissues within that space. This definition brings out the four requisites of a compartmental syndrome: a limiting envelope within which increased tissue pressure produces reduced tissue circulation that results in abnormalities of neuromuscular function.
My sole purpose in using the term "compartmental syndrome" rather than "compartment syndrome" is to indicate that I employ the foregoing definition as opposed to the multiple vague definitions associated with the term "compartment syndrome." The definition permits the development of a "unified concept which is founded on the premise that increased tissue pressure produces similar circulatory and functional effects wherever the process is located and whatever the initiating cause may be. 2l For example, increased tissue pressure in the forearm from a fracture and increased tissue pressure in the leg from intensive use of muscles are seen to produce similar physiological effects and clinical manifestations. Furthermore, the treatment of these two compartmental syndromes is the same: restitution of local blood flow by decompression of the tissues within the compartment.
This unified concept permits us to discuss a wide variety of compartmental syndromes together as a family group, distinguishing among the members only as their individual peculiarities require. Specific etiologies may be indicated, e.g., compartmental syndromes due to intensive use of muscles." Location may also be specified e.g. "deep posterior compartmental syndromes of the leg." Thus the definition of the compartmental syndrome and the unified concept provide an organized system of nomenclature for referring either to all of these conditions as a group or to any member of that group.
In discussing the effects of increased tissue pressure on local circulation in compartmental syndromes the net force per unit area exerted on the walls of vessels is of primary importance.
Pressure measurement techiques
Several methods have been described for the measurement of tissue pressure only a few of which are clinically useful.
The infusion technique is a reliable method for continuously monitoring tissue pressure in the clinical situation.
The continuous infusion and wick techniques give similar pressure readings for intramuscular tissue pressures in animal and human model systems.
Tissue pressure within a limb may significantly exceed the pressure applied externally to the limb.
To be reliable and thus clinically useful any tissue-pressure-measurement technique should be practiced in normal subjects before it is used for evaluation of a patient with a possible acute compartmental syndrome.

Infusion Technique
Definition of tissue pressure
By definition increased tissue pressure is the primary pathophysiological factor in compartmental syndromes. We must therefore define tissue pressure and attempt to resolve some of the confusion that has resulted from previous usage of this term.
A nonhomogeneous and anisotropic material such as tissue cannot be thought of as having a pressure in the same sense as a liquid or gas. This ambiguity is resolved somewhat by considering the two contexts in which the term "tissue pressure" might be invoked. The first concerns the exchange of fluid across a capillary wall. 1 2 This fluid movement is related to:
K (PC - PT ( H) + R OT - R OC) [ 1 ]
where K is a constant PC is the capillary blood pressure PT ( H) is the hydrostatic pressure of tissue fluid R is the capillary membrane reflection coefficient OT is the oncotic pressure of tissue fluid and OC is the oncotic pressure of blood plasma.
The second situation in which the concept of tissue pressure might be invoked is in the consideration of forces operating on a vessel wall. The law of Laplace has been applied to this situation:
PI - PO = T/R [ 2 ]
where PI is the pressure exerted on the inside of the vessel wall PO is the net force per unit area exerted on the outside of the vessel wall T is the tension in the vessel wall and R is the vascular radius.
Neither PT (H) nor PO is simple. Because extracellular fluid may exist in a free form in a gel and perhaps in other forms PT (H) should actually refer to the physical chemical activity of extracellular fluid. 4 By contrast PO is the resultant of several different elements. A positive contribution to PO may result from interstitial fluids gels and matrices as well as from fibers and cells under compression. A negative contribution to PO may arise from cells and fibers under tension. Thus in the general case it cannot be assumed that the two "tissue pressures" [PT (H) from equation 1 and PO from equation 2] are equal. 5- 6 To appreciate how they may differ we have only to consider the analogy of a beaker that contains water and ball bearings. The hydrostatic pressure of water at the bottom of the beaker [analogous to PT(H)] is equal to the height of water in the beaker (H). The net force per unit area on the beaker bottom (analogous to PO) is equal to the total weight in water of the ball bearings (W) divided by the surface area of the beaker bottom (A) plus the hydrostatic pressure: W/A + H.
In discussing the effects of increased tissue pressure on local blood flow the "tissue pressure" of primary interest is PO the net force per unit area exerted on the outside of a vessel wall. This is the force that affects the pressure in and the flow through collapsible vessels. The mechanisms by which increased tissue pressure compromises local blood flow will be discussed in greater detail in Chapter 3.
Tissue pressure measurement
Because tissue pressure plays a central role in compartmental syndromes it is appropriate to review some of the described techniques for tissue pressure measurement.
The capsule method employs a porous capsule surgically implanted in the tissue to be studied. After several weeks the fluid in the capsule reaches equilibrium with the surrounding interstitial fluid. The pressure of the fluid within the capsule is then measured with a pressure transducer. 5- 7- 8 This method has the clinical disadvantages of requiring surgical implantation and a prolonged period for equilibration. Stromberg and Wiederhielm 8 have criticized the capsule method on the basis that the observed pressure is influenced by the osmotic gradient between the fluid inside and the fluid outside the capsule.
Collapsible segment methods measure the pressure inside a flaccid-walled structure located within the tissue. 9 10 These methods are based on equation 2: when the walls of a fluid-filled structure are flaccid (the tension of the walls [ T ] is zero) the pressure of the fluid inside (PI) is equal to the pressure outside (PO). ; Thus the measuring of the fluid pressure inside this structure ; yields the tissue pressure. Although some of these methods have l the disadvantage of requiring surgical implantation Ryder et al. 11 and Kjellmer l2 have described variations using an in situ vein as the collapsible segment. Although the method of Ryder et al may be clinically useful for measuring subcutaneous tissue pressure it is impractical for the measurement of intramuscular pressure in a X traumatized limb because it would require the cannulation of a deep intramuscular vein and repeated raising and lowering of the limb relative to the heart.
A servonull technique with micropipettes has been described by Wiederhielm. 4 In this method no net fluid is injected into the tissue yet a continuous fluid column between the transducer and the tissue is maintained by a servosystem. Although this method is highly accurate and responsive it appears to be too delicate and complicated for routine clinical use.
The injection technique measures the pressure necessary to inject a small quantity of fluid into the tissue through a f needle. l3-l7 Although this method has the advantage of using inexpensive equipment it has a disadvantage in that a steady-state reading is not attained. Thus it may be somewhat awkward in practice because a fluid manometer and an air-water meniscus must be observed simultaneously to detect the pressure at which fluid first begins to flow into the tissue. In an animal model where tissue pressure was elevated by fluid infusion at a known pressure Hargens et al found that the injection technique overestimated low tissue pressures and underestimated high tissue pressures. Clayton et al 9 evaluated the injection technique by applying known pressures to the extremities of six rabbits with a pneumatic cuff. A good linear correlation was obtained with a slope of 1.03 (r = 0.99).
The wick technique employs strands of wettable material extending into the tissue from a fluid-filled catheter connected to a pressure transducer. 6 7 20-22 The wick increases the surface area in contact with the tissue. To protect its fibers the wick catheter is inserted through a larger cannula which is then withdrawn. Clotting around the fibers is minimized by heparinization of the fluid within the catheter. Various materials have been used to make wick catheters; these include cotton and polyglycolic acid suture. The latter is most commonly used in the clinical situation. Zeluff 23 pointed out however that polyglycolic acid suture has a short shelf life after sterilization and suggested that Dacron (DuPont) may be a more suitable material.
Continuity of the fluid column between the tissue and the transducer is necessary for accurate pressure measurement. This continuity may be verified by observing a sharp increment in the observed pressure when the tissue overlying the catheter is pressed manually. If catheter patency cannot be assured the catheter may be flushed with a small volume of heparinized saline. Mubarak et al 22 found that the wick catheter accurately reflected pressures applied by fluid infusion in dog limbs. We obtained reproducible results with the wick catheter when known increments of pressure were applied externally to rabbit and human limbs as long as the wick catheter remained patent. 24
In the continuous infusion technique the patency of a hypodermic needle or intravenous catheter inserted into the tissue is maintained by the slow but continuous infusion of nonheparinized saline solution. The pressure of the fluid within the needle or catheter is continuously monitored with a standard blood pressure transducer. Since its original descriptions this technique has been improved through the use of noncompliant tubing a simplified fluid path and an ordinary needle or catheters
For continuous pressure monitoring an infusion rate of 0.7 cc per day is used. Laboratory studies have demonstrated that the pressure measured is relatively independent of the rate of infusion: an acute 40-fold increase in the infusion rate from 0.7 to 29 cc per day produced only a 4-mm Hg increase in measured pressure. 25 It could be argued theoretically that even a rate of infusion as low as 0.7 cc per day could be hazardous to the patient. For example Hargens et al 2 found that the acute infusion of 2 cc of plasma into a canine anterolateral compartment (volume of 40 cc) raised the intracompartmental pressure from 30 to 45 mm Hg. The pressure increment from saline infusion is unlikely to be a problem clinically however for two reasons: saline is absorbed three times more rapidly than plasma 2 and three days of pressure monitoring would be necessary to infuse the volume of 2 cc. Furthermore most human compartments are well over 10 times as large as the canine anterolateral compartment. The data obtained by Whitesides et al 7 from a limb amputated for sarcoma of the femur indicated that over the range of intracompartmental pressures from 10 to 50 mm Hg the infusion of 1 cc of saline into the anterior compartment of the leg produced a 1-mm Hg increment in intracompartmental pressure. Thus even assuming the worst possible case in which saline absorption is zero (i.e. a totally ischemic compartment) three days of continuous pressure monitoring with an infusion rate of 0.7 cc per day would give rise to an increment in tissue pressure of only 2 mm Hg.
We have demonstrated the accuracy and dependability of the continuous infusion technique in rabbit and human model systems where known increments of pressure were applied to living limbs.
Results of different tissue pressure measurement t
Earlier in this chapter we discussed the fact that there are at least two different "tissue pressures": the PO in the law of Laplace and the PT(H) in the capillary filtration equation. Because these two quantities cannot in the general case be expected to be identical it would not be surprising if different tissue-pressure-measuring techniques yielded somewhat different values.
To determine whether or not the wick and continuous infusion techniques yielded significantly different results we conducted side-by-side studies in rabbit and human model systems in which increments of external pressures were applied. We found that as long as wick catheter patency was closely monitored and any obstruction was cleared by flushing with a minimal volume of fluid the two methods yielded virtually identical results in compressed rabbit and human muscle.
Even when no pressure was applied our side-by-side comparison yielded similar pressure measurement values in human tibialis anterior muscle with the wick and the infusion techniques: 7+3 mm Hg and 9+3 mm Hg respectively. 24 These values do not appear to be significantly different from those obtained with the wick technique by Mubarak et al 22 in normal forearm and leg muscle: 4 +4 mm Hg. Thus in our hands there is no practical difference between the results of the wick and the infusion techniques for intramuscular pressure measurement.
I prefer the continuous infusion technique for clinical tissue pressure measurement for the following reasons:
- No specially prepared catheter is required; any needle or small intravenous catheter will serve. In compartments of the forearm and leg we routinely use a standard 22-gauge intravenous catheter with a 19-gauge inserting needle. This is considerably smaller than the 14- and 16-gauge placement units recommended for use with the wick catheters 22 We have used 25- and 27-gauge needles to measure pressure within the interosseous compartments of the hand.
- Heparinization of the fluid within the catheter is not required; thus the possibility of enhanced local bleeding is eliminated.
- Catheter patency is continuously maintained by a volume-controlled infusion. As a result catheter obstruction has not occurred in our clinical use of this monitoring method. Continuous pressure monitoring may be carried out for periods of at least 72 hours without the need for adjusting or manually flushing the system.
- The pressure can be read at any time from the meter of the transducer monitor.
- The equipment for the technique is available in most hospitals. Anesthesiologists are well acquainted with the calibration zeroing and operation of pressure transducers and can be of great assistance in setting up the system.
- The results are accurate and reproducible.
- On removal of the catheter or needle there need be no concern about retained wick elements. 18
Like all other techniques for tissue pressure measurement the continuous infusion method requires attention to detail and practice before proficiency and the necessary bedside efficiency are attained. Thus I believe it is unwise to try to learn this or any other tissue pressure-measuring technique when confronted with a possible acute compartmental syndrome. The experience is likely to be frustrating and the pressure reading obtained is unlikely to be reliable. An incorrect pressure reading is worse than none at all because it may distract from important clinical findings.
For those interested in being prepared to perform reliable pressure measurements I would suggest practicing the technique on normal subjects until consistent readings in the normal range are obtained. If desired one can apply a known pressure to the limb with an air splint to see if the measured pressure increases by the expected increment.
Relationship of applied and measured pressure
It has been generally assumed that the pressure applied to the outside of a limb is distributed essentially unaltered throughout the tissue. However in our initial studies of the infusion technique 25 we identified a phenomenon of "summation": the intramuscular pressure measured when an external pressure is applied to a limb is equal to the measured intramuscular pressure before pressure application plus the externally applied). More recent data 24 indicated an additional feature in the variance of applied and measured pressure: the increment in measured pressure within muscle exceeds the increment in applied pressure by a factor ranging from 1.02 to 1.3 depending upon the model system as indicated by slopes of the plots of measured and applied pressure. This geometric augmentation of applied pressure is referred to as "amplification." 24 This phenomenon was found to be most striking in the anterior compartment of the rabbit leg where the amplification factor obtained with both the wick and infusion techniques was 1.3 (i.e. the increment in measured pressure was 30% higher than the increment in externally applied pressure). At present we have not identified the mechanism of amplification. The fact ; that in the rabbit anterior compartment the amplification factor was dramatically diminished by fasciotomy suggests that the nonyielding fascia may have a significant effect on the pressure field induced by externally applied pressure.
Both summation and amplification can result in a significant difference between the pressure applied to an extremity and the pressure measured within it. This difference may be important in e interpreting experiments on the amount of pressure required to arrest blood flow 33 in deciding the safe limits for air splints and pressure dressings 34 and in interpreting the results of sphygmomanometry. 35 The magnitude of the apparent discrepancy is significant. The observed relationship of externally applied pressure.
Increased tissue pressure compromises tissue blood flow tissue oxygenation and tissue function.
Reduction in tissue blood flow
Sequential increases in tissue pressure produce increasingly more severe reductions in tissue blood flow and tissue oxygenation. No evidence has been found to support the concept of a "critical pressure" above which the circulation is suddenly compromised.
Increased tissue pressure results in an increase in local venous pressure which produces a diminished local arteriovenous gradient. When increased tissue pressure reduces the local arteriovenous gradient and local blood flow to the point where the metabolic demands of the tissue are no longer met loss of tissue function and thus a compartmental syndrome ensue.
compartmental syndromes
Pathophysiology of increased tissue pressure
The normal function of tissue is maintained by circulation that is sufficient to meet the tissue's metabolic needs. In a compartmental syndrome increased tissue pressure compromises local circulation to the point where the tissue's metabolic needs are no longer met and functional abnormalities ensue. This chapter will first review the data demonstrating that increased tissue pressure compromises tissue circulation oxygenation and function and then proceed to a discussion of the mechanisms by which this pressure-induced circulatory compromise may be produced.
The fact that increased tissue pressure compromises local circulation has been demonstrated by the plethysmographic studies of Ashton. 1 A similar effect has been demonstrated by others who observed the washout rates of various indicators. Rorabeck and Clarke 2 and Rorabeck and MacNab 3 used technetium and xenon Dahn et al 4 used xenon Sheridan et al 5 used rubidium and we 6 have more recently used argon. Taken together these studies indicate that pressure as low as 20 mm Hg applied to a limb can significantly reduce local blood flow. This reduction in blood flow becomes increasingly severe at higher pressures. In none of these studies was there evidence for a "critical" pressure above which tissue blood flow suddenly became compromised.
One of the most important functions of the local circulation is to deliver oxygen to the tissue. The Teflon (Du Pont) membrane catheter-mass spectrometer system is useful for continuously monitoring muscle PO2 which is in turn a reflection of the balance between tissue oxygen delivery and tissue oxygen consumption. 5-9 We conducted studies in rabbit and human model systems correlating anterior tibial muscle PO2 with externally applied and measured tissue pressures. The results were very similar to those of the blood flow studies; that is higher tissue pressures resulted in lower muscle PO2 values 6 8 l0. In the human subjects muscle PO2 was significantly reduced by an applied pressure of 20 mm Hg. Muscle PO2 values decreased essentially linearly as the applied pressure was increased. Thus again no critical pressure was observed but rather a greater compromise of muscle PO2 at higher tissue pressures.
Increased tissue pressure also compromises neuromuscular function. Sheridan et al 5 evaluated the effect of inflation of an intracompartmental latex balloon on the response of nerve and muscle to direct electrical stimulation in a rabbit model system. Higher pressures and longer periods of pressure application produced more frequent functional losses. In another rabbit model system we arrested nerve conduction by applying external pressure to the hindlimb 6. Rorabeck and Clark 2 and Hargens et al 11 slowed nerve conduction velocity by the pressure-controlled infusion of blood or plasma into the anterior compartment of the legs of dogs. We were able to produce similar decrements in human nerve conduction velocity through the external application of pressure to the leg 12.
In theory these pressure-induced functional deficits could be due either to a direct mechanical effect of increased tissue pressure or to reduced tissue circulation. We may argue strongly for the second alternative because the amount of pressure a limb can tolerate before functional abnormalities are produced is altered by factors affecting local blood flow: limb elevation arterial occlusion hypotension and hemorrhaged 13 If the effect of pressure on nerve and muscle were purely mechanical these factors would not be expected to change the pressure tolerance. The concept of tissue pressure tolerance will be discussed in greater detail in Chapter 4.
This review of the physiological effects of increased tissue pressure indicates that tissue circulation is compromised by applied pressures as low as 20 mm Hg and that tissue circulation is increasingly reduced as applied pressure is raised from this value. Any theory concerning the mechanism by which increased pressure affects local circulation must be consistent with these observations. In this light let us review several of the proposed mechanisms of pressure-induced circulatory compromise.
Benjamin 14 Eaton and Green 15 Foisie 13 and Gardner 17 suggested that increased tissue pressure induces arterial spasm which in turn produces intracompartmental ischemia. Although there is some clinical and laboratory evidence to support this mechanism l4' 15 the commonly observed preservation of pulses distal to the affected compartment would tend to refute it. 19-20 Furthermore arteriograms performed on patients with compartmental syndromes usually do not show arterial spasm but rather gradual tapering of the vessels as they course through the affected compartment.
Burton 21-22 and later Ashton 1 observed that as progressively higher external pressures were applied to a limb blood flow ceased before the difference between mean arterial and applied pressure became zero. These observations have given rise to the critical closure theory. According to this theory a significant transmural pressure (mean arteriolar pressure minus tissue pressure) is required to maintain arteriolar patency. This transmural pressure is necessary because of the high tension in the walls of arterioles which is actively produced by smooth muscle contraction. When tissue pressure is elevated to the point that transmural pressure is insufficient the arterioles actively close and blood flow ceases. Support for critical closure is gained from the studies of Ashton 1 on the effect of limb temperature. She demonstrated that the transmural pressure at which circulation ceased varied with limb temperature in a manner consistent with the critical closure theory: a greater transmural pressure was required to maintain blood flow when the tone of arteriolar wall smooth muscle was increased by local cooling. Although critical closure may occur over the short term I doubt that this mechanism could produce prolonged compartmental ischemia because ischemia is a strong local stimulus for vasodilatation. Furthermore in the presence of ischemia there is a diminishing energy supply available for the maintenance of active smooth muscle contraction. l 23
Dahn et al 4 proposed the "tidal wave" theory. They suggested that unless tissue pressure is significantly below arterial diastolic pressure the microcirculation will not remain open long enough to perfuse the capillaries but will rather ebb and flow on the arteriolar side of the microcirculation. While this is a picturesque theory there is as yet insufficient evidence to support it.
Hargens et al 24- 25 made direct measurements of normal capillary pressure and found it to be between 20 and 33 mm Hg. They postulated that when tissue pressure exceeds these values capillary blood flow is reduced by microvascular occlusion. Unfortunately no measurements of capillary pressure have been made in the presence of increased tissue pressure. If one assumes that capillary pressure remains approximately 30 mm Hg when tissue pressure is increased it would seem reasonable to expect these vessels to become occluded if tissue pressure exceeded this level. However venous pressure rises in the presence of increased tissue pressure. 28-23 If venous pressure rises capillary pressure must also rise; thus the 30 mm Hg value for capillary pressure does not appear to be relevant to the situation in which tissue pressure is elevated.
Several investigators including Kjellmer 29 Reneman 30 and Matsen et al 27 31 have proposed what might be referred to as the arteriovenous gradient theory. According to this theory increases in tissue pressure reduce the local arteriovenous gradient and thereby local blood flow. When blood flow is reduced to the point where it no longer meets the metabolic demands of the tissue (but not necessarily to zero) functional abnormalities and thus a compartmental syndrome result.
The relationship between arteriovenous gradient and local blood flow is as follows:
LBF = (PA - PV)/R
where LBF is local blood flow PA is local arterial pressure PV is local venous pressure and R is the local vascular resistance. Because veins are collapsible tubes the pressure inside them (PV) cannot be less than local tissue pressure (PT). Thus when tissue pressure rises the pressure in the local veins must also rise. This increased local venous pressure reduces the local arteriovenous gradient. This phenomenon has been confirmed by the direct measurement of local venous pressure 27-29.
Some reduction in local arteriovenous gradient can be compensated for by changes in local vascular resistance. This process known as autoregulation 33 maintains local blood flow over a range of arteriovenous gradients. However when the arteriovenous gradient is significantly reduced autoregulation becomes relatively ineffective. 23 At this point the local blood flow is determined primarily by the local arteriovenous gradient. With further increases in tissue pressure local blood flow is reduced to the point where it no longer meets the metabolic demands of the tissue functional abnormalities ensue and a compartmental syndrome results.
The arteriovenous gradient theory is consistent with the observed reduction in tissue blood flow accompanying even a small increase in tissue pressure and with the greater reductions in tissue blood flow observed as tissue pressure is further increased. It emphasizes the interrelationships of tissue pressure local venous pressure local blood flow and the metabolic demands of the tissue.
Clinically it is useful because it predicts that a reduction in local arterial pressure (for example from elevation of the limb above the heart) will exaggerate the circulatory effect of any given tissue pressure increase. It further predicts that lowering local venous pressure by decompressing the tissue (lowering local tissue pressure) is an effective method for restoring circulation if a compartmental syndrome ensues. Finally this theory predicts the preservation of pulses and distal circulation frequently seen in a compartmental syndrome. The pulses and distal circulation may remain intact for two reasons. First the increases in tissue pressure usually observed in compartmental syndromes have a minimal effect on arterial flow. Second the venous pressure in the digits distal to the compartment is usually normal; thus a normal digital arteriovenous gradient and normal digital blood flow result.
Tolerance of tissue for increased pressure
Increased tissue pressure compromises nerve and muscle function.
The time required to produce functional abnormalities is related to the severity of the pressure-induced circulatory compromise.
Nerve and muscle have a significant potential for recovery and reconstruction following ischemic injury.
Individuals differ with regard to the amount of pressure their limbs can tolerate before neuromuscular deficits are produced.
Hypotension hemorrhage arterial occlusion and limb elevation all appear to reduce the tolerance of limbs for increased tissue pressure.
In Chapter 3 we saw that increased pressure compromises tissue blood flow tissue oxygenation and tissue function.. In dealing with clinical compartmental syndromes the physician is concerned with the pressure tolerance of the tissue i.e. how much pressure tissue can tolerate before its function becomes abnormal. Abnormal tissue function ensues when local blood flow is reduced to the point where it no longer meets the tissue's metabolic demands.
The pressure tolerance of tissue depends on several factors:
- The specific effect of increased tissue pressure on local blood flow in the tissue under consideration.
- The metabolic demands of the tissue.
- The duration of increased tissue pressure.
Each of these factors may vary from one clinical situation to another. The specific relationship of tissue pressure and tissue blood flow is affected by the presence of hypotension shock arterial occlusion and limb elevation. The metabolic demands of tissue are related to the presence and severity of local tissue injury. Finally the duration of increased pressure depends on the rate of onset of the compartmental syndrome and the promptness with which it is treated. Thus the tolerance of tissue for increased tissue pressure will vary considerably among patients. Because of this variability one specific value for tissue pressure tolerance cannot be applied to the general population. With this perspective let us review some of the specific data available on the tolerance of nerve and muscle for increased tissue pressure in animal and human model systems.
Tolerance of nerve for increased tissue pressure
Several recent studies have investigated the effects of different tissue pressures on nerve function. Sheridan et all inflated a latex balloon within the anterior compartment of rabbits to investigate the effects of different pressures on the response of nerve and muscle to direct electrical stimulation. Only one of the four rabbits receiving a pressure of 40 mm Hg for six hours demonstrated a loss of response to the electrical stimulus. An applied pressure of 60 mm Hg for six hours produced more consistent functional losses. Each rabbit receiving a pressure of 100 mm Hg for 8 or 12 hours lost all discernible response to nerve or muscle stimulation.
In a different rabbit model system in which external pneumatic pressure was applied we investigated the effects of different pressures on the conduction velocity of the tibial nerves. Each pressure tested was applied for a period of five hours. Conduction ceased completely in nine animals: seven of the eight that received 80 mm Hg one that received 70 mm Hg and one that received 60 mm Hg. An average of 2.2+1.6 hours intervened between the application of pressure and the cessation of conduction in these nine animals (range: eight minutes to four hours).
Rorabeck and Clarke 3 investigated the effect of a pressure-controlled infusion of autologous blood into the anterior compartments of dogs. They found that a pressure of 40 mm Hg reduced peroneal nerve conduction velocity from 40 to 30 m/sec over 2.5 hours. A pressure of 80 mm Hg arrested peroneal nerve conduction after four hours.
Hargens et al 4 investigated a model compartmental syndrome in which tissue pressure was elevated by the pressure-controlled infusion of autologous plasma. They found no changes in peroneal nerve conduction velocity when 10 mm Hg was applied for eight hours. Although there was some slowing of nerve conduction when a pressure of 30 mm Hg was applied for 8 hours and when 40 mm Hg was applied for 14 hours neither of these conditions was sufficient to arrest nerve conduction. A pressure of 50 mm Hg exerted for 330 minutes did arrest conduction. Less time was required to arrest nerve conduction at higher tissue pressures: nerve conduction ceased after only 50 minutes when a pressure of 120 mm Hg was applied.
In a human model systems we investigated the effect of different externally applied pressures on the nerve conduction velocity and clinical neurological examinations of three normal subjects. Pressures were applied for a maximum of 80 minutes. Each subject demonstrated a different tolerance for increased tissue pressure. Subject 1 maintained essentially normal function until the tissue pressure (as measured by the continuous infusion technique) exceed 65 mm Hg. Subject 2 tolerated pressures up to 75 mm Hg and subject 3 tolerated pressures only as high as 55 mm Hg. This variability in pressure tolerance could not be attributed to differences in systemic blood pressure. When the pressure tolerance for each subject was exceeded clinical and electrophysiologic changes occurred in a reproducible sequence.
Tolerance of muscle for increased tissue pressure
It is more difficult to quantify the effect of increased tissue pressure on muscle function than on nerve function. For example we have been unable to develop a good model for the measurement of the strength of muscle contraction in a limb with increased tissue pressure. The only studies of muscle function found in the literature are those of Sheridan et all in which the response of muscle to direct electrical stimulation was observed.
Other investigators have correlated the amount and duration of pressure application with evidence of muscle damage. In their model compartmental syndrome Rorabeck and Clarke 3 found increased femoral vein creatinine phosphokinase activity when a pressure of 40 mm Hg was applied to the anterior compartments of dogs. However the absolute value of this enzyme could not be correlated with the amount of pressure applied. Similar findings were noted for lactic dehydrogenase. Hargens et al 7 investigated the effects of increased pressure in their model system using technetium-99m stannous pyrophosphate. They found that in the dog intracompartmental pressures in excess of 20 mm Hg produced a significant uptake of the label when maintained for eight hours. From this point the amount of uptake increased dramatically as higher pressures were applied.
Recovery of nerve and muscle
Both nerve and muscle have the capacity for recovery after a significant ischemic insult. Rorabeck and Clarke 3 studied the recovery of canine nerve function for six hours after surgical treatment of model compartmental syndromes. In these short-term studies they found that if surgical decompression was performed within four hours after the initiation of a compartmental syndrome nerve conduction velocity always returned to normal regardless of the amount or duration of pressure application. If surgical decompression was performed 12 hours after a pressure of 40 mm Hg or more was introduced the nerve conduction velocity did not return to normal within the six-hour period of observation. In his studies of tourniquet palsy in rabbits Lundborg 3 demonstrated that the extent and rapidity of nerve recovery depend on the duration of the ischemic insult. All 10 animals with two hours of nerve ischemia recovered after two weeks. Out of 12 animals with six hours of nerve ischemia only 5 had recovered six weeks later.
Sanderson et al 9 and Vracko and Bendittl indicated that as long as the basal lamina remained intact muscle had a significant potential for reconstruction after an ischemic insult.
Because nerve and muscle have the potential to recover after an ischemic insult caution should be used in applying the term "irreversible" to ischemic damage. For the same reason debridement of potentially viable muscle and nerve at the time of surgical decompression should be kept at an absolute minimum.
Clinical data on pressure tolerance
To help elucidate pressure tolerance in the clinical situation we investigated 42 patients at risk for compartmental syndromes. These patients were frequently examined for clinical evidence of a compartmental syndrome. Tissue pressure in the compartment at greatest risk was continuously monitored with the infusion technique. No patient whose peak pressure was 45 mm Hg or less developed a clinical compartmental syndrome whereas all of those with peak pressures of 60 mm Hg or more did develop a clinical compartmental syndrome. Five of the seven with pressures between 45 and 60 mm Hg developed compartmental syndromes while two did not. Thus these patients varied significantly with regard to their pressure tolerance.
The variability in pressure tolerance among individuals is of particular interest for two reasons:
- It indicates that there is no "critical pressure" that can serve as a general criterion for diagnosis and treatment of a compartmental syndrome.
- It prompts us to investigate the factors responsible for this variability in pressure tolerance to gain a better understanding of the compartmental syndrome.
Factors affecting the pressure tolerance of tissue
The arteriovenous gradient theory for the pathogenesis of a compartmental syndrome (see Chapter 3) enables us to predict that any cause of lowered local arterial pressure will diminish the pressure tolerance of tissue. We have investigated the effects on pressure tolerance of four clinically common causes of reduced local arterial pressure: anesthetic-induced hypotension hemorrhagic shock arterial occlusion and limb elevation. In a rabbit model system we investigated the effect of hypotension induced by halothane anesthetic on muscle blood flow and muscle PO2. 2 A pressure of 60 mm Hg was applied to a hindlimb in two groups of animals: one in which the halothane anesthetic used for preparation was discontinued immediately after preparation and a second in which the halothane anesthetic was continued throughout the entire five-hour period of pressure application. The circulatory effect of 60 mm Hg was much more profound in the hypotensive animals.
In a second study we investigated the effect of hemorrhagic shock in a rabbit model systems This condition is particularly important clinically because individuals sustaining compartmental syndromes from trauma may also sustain hemorrhagic shock. We investigated the effects on the pressure tolerance of rabbit hindlimbs of an acute 20% hemorrhage produced by the removal of 12 cc of whole blood per kilogram. This 20% hemorrhage reduced mean arterial blood pressure from a mean value of 88_12 to 75_1 1 mm Hg. An applied pressure of 40 mm Hg led to significantly greater reductions in muscle nitrogen washout muscle oxygenation and muscle action potential amplitude in the hemorrhage group as compared with the nonhemorrhage group. 11
Zweifach et al 12 investigated the effect of hemorrhage on the uptake of technetium-99m after the application of different intracompartmental pressures in dogs. In this study some animals were bled until mean arterial pressure reached 65 mm Hg. An applied pressure of 25 mm Hg produced the same effect in the hypotensive animal that a pressure of 40 to 50 mm Hg produced in a normotensive animal.
We carried out a study of the effect of superficial femoral arterial ligation on the response of rabbit muscle to an applied pressure of 40 mm Hg l3. Although arterial ligation alone produced significant decrements in muscle nitrogen washout PO2 and action potential amplitude all of these parameters fell to zero when the external pressure of 40 mm Hg was applied.
We also manipulated the pressure tolerance of normal human limbs. Instead of inducing systemic hypotension by hemorrhage or anesthetic we induced local arterial hypotension by elevating the limbs above the level of the heart. Limb elevation reduced local arterial pressure by an amount equal to the pressure produced by the column of blood from the limb to the heart. This hydrostatic column pressure may be calculated by dividing the amount of limb elevation above the heart in centimeters by 1.3 cm of whole blood per millimeter of mercury l3-14.
Elevation of a limb from the dependent position may lower local venous pressure; however elevation cannot lower local venous pressure below the level of local tissue pressure. Thus for any given tissue pressure elevation of a limb above the supine position reduces the local arteriovenous gradient. The diminished pressure tolerance of elevated limbs was apparent in our series of experiments in which anterior compartment muscle PO2 was measured in normal subjects l5. A pressure of 20 mm Hg applied to a limb elevated 54 cm above the heart reduced muscle PO2 by the same amount as a pressure of 60 mm Hg applied to the limb level with the heart.
We demonstrated the functional significance of this lowering of pressure tolerance by limb elevation in another series of experiments. 5 Here nerve conduction velocity was measured in level and elevated limbs receiving different applied pressures. Once more the tolerance of the limbs for increased tissue pressure was diminished by an amount equal to the hydrostatic column pressure produced by the limb elevation.
These investigations of elevated limbs are clinically important in that they suggest that limbs with compartments showing signs of inadequate blood flow should not be elevated. In this situation elevation will lower local arterial pressure but will not affect local venous pressure. Thus the arteriovenous gradient will be further diminished when blood flow is already inadequate. These findings have proved clinically useful: on several occasions the symptoms and signs of compartmental syndromes have resolved upon lowering the affected limbs from an elevated position. Unnecessary surgical decompression has thereby been avoided.
Etiologies of compartmental syndromes
A compartmental syndrome may occur whenever tissue pressure within a limited space rises to the point that it compromises local circulation and function.
The two prerequisites for a compartmental syndrome are (a) a limiting envelope surrounding tissue and (b) a cause of increased tissue pressure within that envelope.
A wide variety of etiologies may produce a sufficient increase in local tissue pressure to cause a compartmental syndrome.
The relative frequency of the different etiologies may differ dramatically in different patient populations.
Postischemic swelling is a particularly sinister cause of a compartmental syndrome.
By definition a compartmental syndrome is produced when the tissue pressure within a limited space rises to the point where the circulation and function of the tissues within that space are compromised.
There are therefore two prerequisites for the production of a compartmental syndrome: (a) an envelope limiting the available space and (b) a cause of increased pressure within that envelope.
The first prerequisite a limiting envelope may be any structure of limited compliance that surrounds tissue. Several different materials may compose these limiting envelopes. Envelopes may consist of fascia and bone as in the anterior compartment of the leg or may consist of fascia alone as in the gluteal compartment. l The skin may serve as a limiting envelope in burned extremities or in cases in which the skin has been closed after surgical opening of the fascia. 2-7 Even the connective tissue layer that surrounds each muscle the epimysium may serve as the limiting envelope in a compartmental syndrome. 8- 9 Limiting envelopes may also be produced by the physician in the form of tight external dressings or casts. In 1881 Volkmann provided one of the first written descriptions of circulatory compromise from tight dressings. His identifications of externally applied pressure as a cause of muscle ischemia is important even though he incorrectly attributed the ischemia to arterial occlusion. Edgar Bick's translation of this description is reproduced below: 10
For many years I have noted on occasion following the use of bandages too tightly applied the occurrence of paralysis and contraction of the limb not as has been previously assumed due to paralysis of the nerve by pressure but as a quick and massive disintegration of the contractile substance and the effect of the ensuing reaction and degeneration. The paralysis and contracture are to be understood as purely myogenic.
A series of new experiences has merely confirmed the correctness of this assertion and also produced certain views about the character of the process here in question. Accordingly I might summarize my views in the following sentences:
- The paralyses and contractures appearing after too tight bandaging of the forearm and hand less frequently in the lower extremity are to be considered ischemic. They are caused by prolonged blocking of arterial blood. The almost simultaneous occurrence of massive venous stasis manifests itself at the beginning of the paralysis only to accelerate its progress.
- The paralysis is based upon the fact that the muscle bundles too long deprived of their acids become necrotic. The contractile substance coagulates disintegrates into clumps and will be resolved later. The ensuing contracture is thereby to be understood above all as simply rigor mortis and shows the paralyzed and contracted limb-if as usual the entire musculature of a limb or part of a limb is affected-always in the same position which we find in the limbs of rigor mortis.
- Characteristically the paralysis and contracture appear simultaneously or follow immediately after one or the other while in paralysis of nerve origin in the extremity the contracture develops gradually and often much later; months and years pass before a deformity develops that cannot be overcome by immediate passive hand-power.
- On the contrary ischemic contracture shows its nature from the first moment by the great resistance it opposes to straightening the limb. The affected muscles have already completely and immediately lost their elasticity as in rigor mortis and are completely stiff.
- The reactive and regenerating processes always very imperfect in man following the disintegration of the contractile substance make the diseased muscles even more unyielding and further increase the contracture by cicatrization.
- Ischemic paralysis and contraction of similar character also occur after application of any tight bandage too long continuation of an Esmarch constriction of the limbs and also after lacerations and contusions of large vessels and perhaps also after long periods of severe cold.
The second prerequisite for a compartmental syndrome a cause of increased pressure within the envelope may be a decrease in the volume of the envelope an increase in the content within the envelope or the application of pressure to the outside of the envelope. Whitesides et al 11 and Hargens et al 12 sequentially increased the content of a dog's anterior compartment while observing the resulting changes in intracompartmental pressure. The initial increases in compartmental content produced only small increments in intracompartmental pressure; thus lax fascia appears to have a significant compliance. With increasing compartmental content intracompartmental pressure rose more steeply that is the fascial compliance progressively diminished. This pressure content relationship is further emphasized by data of Whitesides et al 11 from an amputated human leg: a 30% increase in the content of the anterior compartment from 110% to 140% of normal raised the intracompartmental pressure only 20 mm Hg (from 10 to 30 mm Hg) whereas a 30% increase in content from 150% to 180% of normal caused the intracompartmental pressure to rise 75 mm Hg (from 45 to 120 mm Hg).
Any cause of locally increased tissue pressure is a potential cause for a compartmental syndrome:
Decreased compartmental volume:
- closure of fascial defects
- application of excessive traction to fractured limbs.
Increased compartmental content:
- bleeding
- vascular injury
- bleeding disorder
- anticoagulants
- increased capillary filtration
- increased capillary permeability
- post ischemic reperfusion
- trauma
- intensive use of muscles
- burns
- intraarterial drugs
- cold
- surgery
- snakebites
- increased capillary pressure
- venous obstruction
- diminished serum osmolarilty
- nephrotic syndrome
- infiltrated infusions
- muscle hypertrophy
- popliteal cysts
Externally applied pressure:
- tight casts dressings
- air splints
- lying on limb
The relative frequency of these different etiologies may vary markedly from one geographical location to another. For example in our series from the University of Washington affiliated hospitals l3 extremity trauma was the etiology in 24 of 44 cases. By contrast in the series from the University of California in San Diego limb compression in association with drug overdose accounted for 5 of 11 cases l4 an etiology not seen in our series.
Whereas the mechanism by which most of the etiologies produce increased tissue pressure is apparent postischemic swelling deserves some additional discussion. Like other tissues capillary endothelium is damaged by prolonged ischemia. This damage is reflected by an increase in capillary permeability. If the circulation is restored through ischemically damaged capillaries the increased capillary permeability results in extravasation of fluid with an increase in extracellular volume. Cell volume may also be increased because ischemia may deprive cells of their normal membrane integrity and ionic pump functions. This postischemic swelling has been demonstrated by two laboratory investigations. Fuhrman and Crismon 83 measured the water content of rabbit muscle two hours after different periods of tourniquet ischemia. They found that three hours of ischemia gave rise to postischemic swelling of 30 to 60%. Whitesides et al 11 measured tissue pressures in the compartments of dog hindlimbs after a period of tourniquet ischemia. The postischemic increment in pressure was higher the longer the tourniquet had been applied. Whereas only a few of the animals with four hours of ischemia showed a significant increase in tissue pressure most of the six-hour and all of the eight-hour animals showed significant pressure increases after the release of the tourniquet.
Compartmental syndromes resulting from postischemic swelling can present a diagnostic challenge. Because the tissue is injured by the initial period of ischemia neuromuscular function may already be abnormal. Thus the detection of additional deficits from a superimposed compartmental syndrome requires very close observation of the patient's nerve and muscle function.
Where do compartmental syndromes occur?
A compartmental syndrome may occur wherever tissue is surrounded by a limiting envelope.
Certain factors may favor the development of a compartmental syndrome in a specific location. Examples include relatively noncompliant fascia exposure to trauma or ischemia and vigorous use of the compartmental musculature.
The frequency with which the different compartments are involved may vary from one geographical area to another.
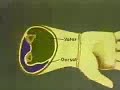
compartmental syndromes
Anatomical locations of compartmental syndromes
A compartmental syndrome may potentially occur wherever a limiting envelope surrounds neuromuscular tissue. Certain anatomical locations are particularly predisposed to the development of a compartmental syndrome. This predisposition may result from the limited compliance of the compartment. Whitesides et also found the human anterior compartment of the leg to be significantly less compliant than the superficial or deep posterior compartments of the leg. A high susceptibility to trauma may be another predisposing factor. For example the anterior compartment of the leg is vulnerable to contusion and is frequently injured in fractures of the tibia. The four compartments of the leg are often affected by ischemic conditions of the lower extremity a situation that places them at risk for compartmental syndromes resulting from postischemic swelling. The muscles of the leg and forearm are often exercised vigorously; thus their compartments are potential sites of compartmental syndromes from intensive use of muscles. Additionally other factors predispose the compartments of the upper and lower extremities to the development of compartmental syndromes including their accessibility for drug injection and their vulnerability to burns.
The relative frequency of involvement of different compartments may vary from one geographical area to another. The high incidence of the anterior compartmental syndrome of the leg in the University of Washington series is a reflection of the large number of trauma cases seen in our hospital system. The relatively high incidence of involvement of the gluteal quadriceps biceps and deltoid compartments in the series from the University of California at San Diego is related to the frequency with which drug overdosage with limb compression is seen there.
Each of most commonly involved compartments is surrounded by relatively unyielding fascia and is in a location where it is predisposed to trauma and other causes of tissue swelling that could give rise to a compartmental syndrome.
Articles of interest on Compartmental Syndromes (PDF) (1.78 MB)
How are compartmental syndromes diagnosed?
Most compartmental syndromes may be diagnosed on the basis of clinical symptoms and signs alone. These include:
- pain out of proportion to what is anticipated from the clinical situation
- weakness of the muscles in the compartment
- pain on passive stretch of the muscles of the compartment
- hypesthesia in the distribution of the nerves coursing through the compartment and
- tenseness of the compartmental envelope.
In certain instances adjunctive diagnostic techniques such as tissue pressure measurement and direct nerve stimulation may be useful in the diagnosis of compartmental syndromes.
The period of risk for compartmental syndromes appears to extend at least to three and possibly to six days after the initial cause of compartmental swelling.
Arterial occlusion and primary nerve injury may produce a clinical picture similar to that of a compartmental syndrome; yet the differential diagnosis can usually be made by careful clinical examination with occasional recourse to ancillary diagnostic techniques.
Videos
Click to play
Anterior compartment of the leg
of the leg

Infusion Technique

compartmental syndrome
Clinical diagnosis
The essential elements in diagnosing a compartmental syndrome are revealed in its definition: a compartmental syndrome is a condition in which increased pressure within a limited space compromises the circulation and function of the contents of that space. Thus to make a rigorous diagnosis of this condition the physician should have evidence for increased tissue pressure inadequate tissue perfusion and loss of tissue function.. When all of these are present the diagnosis of a compartmental syndrome may be made with assurance; when one or more of these factors is absent the diagnosis is less secure.
Evidence for increased tissue pressure may include the patient's complaints of tightness or pressure in the involved area. The physician may perceive tenseness of the compartmental envelope by palpation. Or he may detect significantly increased tissue pressure by direct pressure measurement.
Evidence for inadequate perfusion of local tissue may include the symptom of pain out of proportion to what would be anticipated from the clinical situation. For example one would not anticipate a progressive increase in pain from a properly splinted fracture. Requests by the patient for more analgesic medication are often discounted by nurses and physicians but may actually provide a vital clue to the onset of locally insufficient blood flow. Pain on stretch of the intracompartmental muscles is a useful indication of inadequate local perfusion particularly if these muscles have not been otherwise injured.
Although muscle blood flow may be quantitated in the laboratory with various measurement techniques these techniques are as yet difficult to apply to the clinical situation. Even if such quantification were practical the results would only be useful if the circulatory requirements of the tissue in question were known.
Peripheral pulses are frequently normal in compartmental syndromes because intracompartmental pressures are usually insufficient to affect arterial flow. Thus whereas diminished pulses suggest reduced arterial flow from some cause or other the presence of distal pulses provides no information about the adequacy of compartmental perfusion. A similar statement may be made about the presence of Doppler signals distal to the compartments In our investigations of a model compartmental syndrome in humans we found that an excellent Doppler signal could be detected in the presence of severely compromised compartmental function.
One may reasonably ask whether compromised tissue perfusion may be determined from tissue pressure measurements alone. Whereas intramuscular pressures in excess of 20 mm Hg are abnormal and have been shown to reduce tissue blood flow and oxygenation 5 6 they do not necessarily indicate inadequate tissue perfusion. The local circulatory effect of a given tissue pressure depends upon the pressure tolerance of the tissue (see Chapter 4). However a rough guideline may be derived from our past experience with clinical tissue pressure monitoring: significantly compromised tissue perfusion is likely when tissue pressure exceeds 45 mm Hg.
Evidence for abnormal tissue function includes weakness of the intracompartmental muscles and hypesthesia in the distribution of nerves coursing through the involved compartment. Because both nerve and muscle function may be altered by direct injury evidence of progressive functional losses after an initial injury is a particularly important sign of a compartmental syndrome. Detection of this progression is obviously dependent upon good neuromuscular examinations repeated frequently and documented adequately.
The function of muscles at risk is graded on a zero to five scale (where zero indicates no function and five indicates normal function). Toe extension must be specifically examined because a patient without any anterior compartment function can "wiggle his toes" quite well by using his toe flexors and then allowing his toes to spring back to the neutral position. Sensation is a bit more difficult to quantitate but most observers could agree on definitions of normal slightly diminished significantly diminished and absent.
It is important to record the time and results of these examinations so that changes in the patient's condition may be easily determined. A shorthand notation is useful.
In most cases the diagnosis of a compartmental syndrome can be made from the clinical evaluation alone. The symptoms and signs usually associated with a compartmental syndrome may be summarized as follows:
- Pain out of proportion to what is anticipated from the clinical situation.
- Weakness of the muscles in the compartment.
- Pain on passive stretch of the muscles in the compartment.
- Hypesthesia in the distribution of the nerves coursing through the compartment.
- Tenseness of the compartmental envelope.
Adjunctive diagnostic techniques
Although the clinical examination is the cornerstone of the diagnosis of compartmental syndromes it has two distinct disadvantages: (a) it is partially subjective and (b) it requires cooperation from the patient. Furthermore in certain situations the clinical evaluation may be insufficient to allow the examiner to distinguish among several possible causes of neuromuscular deficit. In these instances quantitative objective techniques such as tissue pressure measurement and direct nerve stimulation may be useful adjuncts.
Tissue Pressure Measurement
Tissue pressure measurement may be of great value in the diagnosis of compartmental syndromes because it quantitates the physical factor responsible for the syndrome. A tissue pressure in excess of 45 mm Hg is usually associated with a compartmental syndrome and a tissue pressure of 60 mm Hg or higher consistently gives rise to this condition. Because the tolerance of tissue for increased pressure may be reduced by such factors as shock arterial occlusion and limb elevation compartmental syndromes may occur at significantly lower tissue pressures (see Chapter 4).
Tissue pressure measurement is most often useful where the diagnosis of a compartmental syndrome cannot be established or excluded on the basis of symptoms and signs alone. The clinical presentation is likely to be ambiguous in a patient who has more proximal neurologic lesions involving peripheral nerves or the central nervous system a patient with other causes of compartmental ischemia or a patient with such anxiety that the usual tests for compartmental function are unreliable. (Even in these situations however the clever clinician is sometimes able to make use of withdrawal reflexes or Babinski signs to evaluate compartmental function.)
Another application of pressure monitoring is in the early detection of compartmental syndromes in patients at risk for this condition. The pressure is continuously monitored in the compartment judged to be at highest risk (the one that is clinically tightest the one that has received the most direct trauma or the one known to be most predisposed to the development of compartmental syndromes). Pressure monitoring is continued until the question of a compartmental syndrome is resolved-a period that usually does not extend beyond three days. The infusion technique is of particular value in this application because it allows continuous pressure monitoring for extended periods.
A typical example of the usefulness of continuous pressure monitoring is the case of a 22-year-old man whose leg had been pinned for five hours beneath a heavy sign. Continuous monitoring of the pressure within the anterior compartment indicated a rise in tissue pressure from 20 to 50 mm Hg in the first two hours after the patient's admission to the hospital. This rapid pressure increase heralded the onset of a compartmental syndrome which was successfully treated by prompt surgical decompression.
The use of tissue pressure measurement in the diagnosis of compartmental syndromes assumes that the measured pressure accurately reflects the pressure within the compartment. There is always a danger particularly in inexperienced hands that the pressure reading is erroneous due to such factors as an occluded catheter a leaky connector bubbles in the system an inaccurately zeroed or calibrated transducer incorrect catheter or needle placement or misreading of the transducer monitor. Bleeding from the catheter insertion may falsely elevate local tissue pressure particularly if a heparinized saline solution is used to flush the catheter. Finally it must be remembered that tissue pressure cannot be measured in all parts of all compartments at risk. Thus a sampling problem may exist: the maximum tissue pressure may be at some point other than where the tissue pressure is being measured. These potential sources of measurement error along with the observation that pressure tolerance varies among individuals indicate that the diagnosis of a compartmental syndrome cannot be based on pressure measurements alone.
Direct Nerve Stimulation
Occasionally one encounters a patient who after an injury is totally unable to contract the muscles within a compartment. The question then arises Is the paralysis due to a primary nerve injury or to a compartmental syndrome? In cases where the patient is unable to voluntarily contract the intracompartmental muscles direct stimulation of the principal motor nerve of the compartment at a point just proximal to the compartment may provide information useful in distinguishing a compartmental syndrome from a more proximal nerve injury. 3 Because the myoneural junction is the part of the motor unit most sensitive to ischemia 9- SO the muscles of a compartment paralyzed by a severe compartmental syndrome would not respond to stimulation of the motor nerve. A normal motor response to the stimulation of the compartment nerve supply would indicate that the cause of the paralysis is not a compartmental syndrome. This type of nerve stimulation requires an inexpensive battery-powered nerve stimulator the type used by anesthesiologists to evaluate the status of the myoneural junction. The stimulus is easily applied by connecting the leads of the stimulator to two long 25-gauge needles sterilely inserted near the nerve in question.
Time at risk
In considering patients at risk for a compartmental syndrome one may appropriately ask How long must the vigil be maintained? In our review of patients having surgical decompression for compartmental syndromes the interval between the etiological event (e.g. contusion or fracture) and the onset of the compartmental syndromes (that is the earliest evidence of functional deficits related to the compartmental syndrome) averaged 15 hours. In our series of patients with deep posterior compartmental syndromes of the leg l2 this interval ranged from two hours to six days with mean and median values of approximately 1% days. In the latter series the most rapid onset of a compartmental syndrome occurred in a 20-year-old male who sustained a severe contusion of his leg that was followed two hours later by deep and superficial posterior compartmental syndromes. The longest interval between the etiological event and the onset of a compartmental syndrome was six days. This occurred when anterior and deep posterior compartmental syndromes resulted from a compound fracture of the distal tibia and fibula.
In an unpublished study Veith l3 prospectively monitored the anterior compartmental pressures in eight patients with displaced closed fractures of the tibial shaft. In each case he found that maximum pressure occurred 21 to 36 hours after the tibial fracture. None of these patients developed compartmental syndromes.
Because the time at risk for a compartmental syndrome extends to three and possibly six days after a significant extremity injury the physician cannot relax his watch until the intracompartmental swelling has shown definite signs of resolution.
Differential diagnosis
Acute arterial occlusion whether from arterial embolization or thrombosis may mimic a compartmental syndrome by producing signs of compartmental ischemia and loss of neuromuscular function. In the case of isolated arterial occlusion local tissue and venous pressures are normal. If increased tissue pressure additionally compromises compartmental blood flow the patient has a superimposed compartmental syndrome. In this case the patient will benefit from surgical decompression. This procedure will improve the local arteriovenous gradient by lowering tissue pressure and local venous pressure.
When faced with a compartmental syndrome and a possible coexistent arterial injury it is usually prudent to perform a surgical decompression immediately. Then if a significant arterial injury cannot be excluded an arteriogram can be performed while the patient is still on the operating table so that prompt vascular repair may be carried out if needed. Arteriography performed before the patient is taken to the operating room may excessively delay surgical decompression.
Primary nerve injury may also present a problem in differential diagnosis. Nerve injury is expected to produce deficits in neuromuscular function but these should not be progressive after the initial injury. Furthermore signs and symptoms of ischemia and increased tissue pressure should be absent. Direct nerve stimulation as described above and standard nerve conduction velocity measurements may be useful diagnostic adjuncts. Electromyography is not likely to be helpful because several weeks are required before signs of denervation manifest themselves.
Other differential diagnostic possibilities include osteomyelitis synovitis tenosynovitis and deep vein thrombosis each of which may produce significant local swelling. A compartmental syndrome may be excluded if neuromuscular function is normal. However it must be remembered that any condition that produces significant intracompartmental swelling may produce a compartmental syndrome.
The most challenging differential diagnostic problems occur when several potential causes of functional loss exist. An example is the loss of anterior compartmental function after an osteotomy of the tibial shaft to correct a valgus deformity. This functional loss could result from (a) a compartmental syndrome (b) a traction injury to the peroneal nerve or (c) a traction injury to the anterior tibial artery. l4.
Details about treatment
The objective of treatment of a compartmental syndrome is to minimize deficits in neurological function by promptly restoring local blood flow usually by surgical decompression.
Certain nonoperative measures may be effective such as eliminating external envelopes and maintaining local arterial pressure.
Vasodilator drugs or sympathetic blocks appear to be ineffective in the treatment of compartmental syndromes probably because in this condition maximal local vasodilatation is already present.
Surgical decompression of all limiting envelopes is usually indicated in the presence of (a) a characteristic clinical picture of a compartmental syndrome or (b) an ambiguous clinical picture in the presence of a measured tissue pressure in excess of 40 mm Hg provided the patient has a normal pressure tolerance.
Only obviously nonviable tissue is debrided at the time of surgical decompression.
The skin is left open after surgical decompression to prevent it from becoming a limiting envelope during the anticipated period of postischemic swelling.
Skin closure may usually be accomplished three to five days after surgical decompression by direct suture or meshed skin graft. The skin may also be progressively closed over the ensuing 10 to 14 days with suture or sterile paper tape.
Skeletal fixation is a useful adjunct to management of the limb when a compartmental syndrome is associated with an unstable fracture.
Increased tissue pressure is the pathogenic factor in the compartmental syndrome. Thus the primary goal in treating this condition is the prompt lowering of tissue pressure to normal levels. A definitive reduction in tissue pressure is accomplished by the complete opening of all envelopes surrounding the affected tissue. This opening must not only decompress the contents of the compartment but also accommodate any postischemic swelling occurring after the decompression procedure. If significant postischemic swelling occurs within incompletely opened envelopes a "rebound" compartmental syndrome may occur.

anterior compartmental syndrome

anterior compartmental syndrome
Opening external envelopes
Because tissue pressure may be increased as a result of tight external envelopes (e.g. dressings and casts) it is essential that such envelopes be eliminated at the first evidence of a compartmental syndrome. Pliable dressings are simply divided down to the level of the skin. Rigid dressings such as casts should be bivalved so that the anterior half may be completely removed. A single cut through a cast even if the cast is spread and wedged open often does not sufficiently increase the volume of the cast envelope. Although removal of the front half of a cast may jeopardize the reduction of a fracture restoration of local circulation must take precedence. Fracture reduction can usually be regained; however local circulatory insufficiency may produce permanent deleterious effects.
Maintaining local arterial pressure
Before operative methods for reducing tissue pressure are discussed the importance of maintaining local arterial pressure should be considered. Local arterial hypotension reduces the tissue's pressure tolerance and increases the adverse effects of a given tissue pressure (see Chapter 4). This is true whether the local arterial pressure has been reduced by shock peripheral vascular disease or elevation of the limb above the heart. Thus treatment of systemic hypotension and avoidance of limb elevation are important for the maintenance of local arterial pressure and in the management of compartmental syndromes.
Although it may seem that vasodilator drugs or sympathetic blocks might also be of benefit in improving local circulation the ineffectiveness of these treatments has been revealed by clinical experience Apparently the local circulatory insufficiency in a compartmental syndrome is such a potent stimulus for vasodilatation that the elimination of sympathetic tone does not additionally augment local blood flow.
Indications for surgical decompression
If the release of all external envelopes and optimization of local arterial pressure fail to eliminate the compartmental syndrome prompt surgical decompression must be considered. Rigid indications for surgical decompression are difficult to establish; each patient and each compartmental syndrome has an individuality that affects the way in which they are managed. In general however surgical decompression is indicated in the presence of:
- Significant deficits in neuromuscular function related to increased tissue pressure. The term "significant deficits" refers to any functional losses that would not be acceptable in the end result. The presence of increased tissue pressure may be detected by palpation of the compartment or by measurement of intracompartmental pressure.
- An ambiguous clinical picture with a tissue pressure above 40 mm Hg in a patient expected to have a normal pressure tolerance. Forty millimeters of mercury is an empirically derived figure based on our experience with prospective monitoring of tissue pressure in patients at risk for compartmental syndromes (see Chapter 4). This value is not proposed as a "critical" pressure applicable to all patients. Patients with peripheral vascular disease patients in shock and patients with elevated limbs are expected to have a diminished pressure tolerance and may require surgical decompression at lower tissue pressures. In arriving at the appropriate therapeutic decision we use pressure measurement data as an adjunct to whatever clinical information is available: the greatest weight is given to the presence severity and time-course of deficits in the function of intracompartmental nerves and muscles.
When indicated surgical decompression is an emergency because delay increases the damage inflicted on intracompartmental tissue as well as the incidence of complications (see Chapter 9).
Techniques of surgical decompression
Several principles are applicable to the surgical decompression of all acute compartmental syndromes. The procedure is performed without a tourniquet to avoid prolonging the period of ischemia and to permit the surgeon to assess the degree to which the local circulation is restored by decompression. Each potentially limiting envelope including skin is opened over the entire length of the compartment; all muscle groups should be soft to palpation at the end of the procedure. If muscle tenseness remains after the skin and fascial incisions have been made epimysiotomy may be required to complete the surgical decompressions The debridement of muscle is kept at a minimum at the time of surgical decompression unless there is obvious muscle necrosis. Muscle that is not contractile at the time of surgical decompression may still have significant potential for recovery or reconstruction. 7 8 Postischemic swelling is likely to occur for several hours after surgical decompression. 9 Therefore the skin is left wide open to prevent the development of a "rebound" compartmental syndrome with the skin as the limiting envelope. Skimping on the length of the skin incision or attempting primary skin closure to improve cosmesis is obviously poor economy if the tissue is inadequately decompressed.
Decompression of the Leg
When a compartment of the leg is involved with an acute compartmental syndrome it is usually preferable to open all four compartments through a single lateral incision without removing the fibula. 11 Because all four compartments are usually exposed to the same etiological events involvement of one compartment may be associated with impending involvement of the others. I have seen two cases in which decompression of only the anterior compartment left the patient with sequelae of an untreated deep posterior compartmental syndrome.
Decompression of the Volar Forearm
The superficial and deep volar compartments of the forearm are easily decompressed through a longitudinal ulnar incision. 11 This procedure is usually combined with section of the transverse carpal ligament. The incision is readily extendible up the arm if further access to the brachial artery is needed.
Care After Surgical Decompression
Sterile dressings are applied followed by splinting to hold the extremity in a functional position. Passive stretching exercises are instituted to maintain the range of joint motion. The patient is returned to the operating room for wound inspection three to five days after surgical decompression. Any obviously devitalized material is debrided at this time although debridement is usually not necessary when decompression has been performed early. The wound is then closed by suture if it is possible to approximate the skin edges without tension. Otherwise one may use a split-thickness skin graft 0.012 in. thick that has been meshed at a 1:1.5 ratio. This meshed graft requires a smaller donor area than a conventional skin graft provides excellent drainage and results in satisfactory cosmesis. When optimal cosmesis and quality of skin cover are desired one may progressively approximate the wound edges over 7 to 14 days with suture or sterile paper tape. 12
Skeletal fixation of associated fractures
Increased tissue pressure within the fascial compartments may splint a fractured limb by an action resembling that of an air splint. When surgical decompression is carried out this splinting effect is lost and the fracture may become considerably less stable.
If a stable and satisfactory reduction cannot be accomplished consideration should be given to skeletal fixation of unstable fractures associated with compartmental syndromes. External pin fixation plates and intramedullary nails have been used in this applications 11 If employed the stabilization is performed immediately after surgical decompression. The management of wound limb and fracture is thus greatly facilitated.
Sequelae of compartmental syndromes
Sequelae of compartmental syndromes may include persistent hypesthesia and dysesthesia persistent motor weakness infection myoglobinuric renal failure contractures amputation and death.
These sequelae are the direct result of nerve and muscle injury and death and thus their frequency and severity are minimized by prompt diagnosis and treatment of compartmental syndromes.
The potential sequelae of a compartmental syndrome include persistent hypesthesia and dysesthesia persistent motor weakness infections of bone and soft tissue renal failure contractures amputation and death. Early treatment of compartmental syndromes is the best method for preventing these sequelae. This point is well demonstrated by our retrospective review of 46 extremities in 44 patients having surgical decompression of compartments afflicted with compartmental syndromes. 1 Only 7 of the 22 extremities decompressed within 12 hours of the appearance of the compartmental syndrome showed residual deficits at the time of follow-up examination; only 1 had a significant complication. In four of these cases compartmental syndromes developed after intra-arterial drug injection a situation in which microembolization also compromises local circulation. 2-4 If these four cases are excluded residual deficits occurred in only 3 of the remaining 18 extremities that received early decompression. By contrast 22 of the 24 extremities having late decompression showed residual functional losses (none of these compartmental syndromes resulted from intra-arterial drug injection). In the late decompression group 13 of the 24 extremities had complications 5 of which required amputation.
It is important to realize that in this study the duration of the compartmental syndrome before surgical decompression was determined retrospectively from the time that the earliest evidence of functional deficits appeared not the time at which the syndrome was diagnosed by the physician caring for the patient. In many cases much of the apparent 12-hour "grace period" had elapsed before the diagnosis of a compartmental syndrome was made.
Motor deficits resulting from a compartmental syndrome are initially treated with appropriate orthotic devices e.g. a drop foot brace when the anterior compartment of the leg is affected. If function does not return in about one year tendon transfer and other forms of reconstructive surgery may be considered. Hypesthesia and painful dysesthesia can also result from a compartmental syndrome. These may resolve slowly with time. Diphenylhydantoin (Dilantin Parke-Davis) and carbamazepine (Tegretol Ciba-Geigy) may be of some value in making the patient more comfortable.
Infection can be a serious complication of a compartmental syndrome. In our retrospective reviews 11 of 24 extremities having late surgical decompression developed infections. Five or almost one-half of these infections led to an amputation. One case of osteomyelitis occurred in a patient with an initially closed tibial fracture who underwent fasciotomy and primary closure 28 hours after the onset of the compartmental syndrome. Infection appears to be most frequent in the presence of devitalized muscle particularly if skin closure has been attempted. Infected compartments are treated by wide opening of dressings skin and fascia thorough lavage of all affected areas and debridement of infected tissue. The wound is treated open with damp dressings until it is sufficiently clean for closure or skin grafting. In some cases of refractory osteomyelitis associated with severe functional losses amputation may present the only reasonable treatment.
Myoglobinuric renal failure is another serious and potentially fatal complication of compartmental syndromes. 5-l0 Myoglobin is released from damaged muscle cells in amounts related to the severity of the muscle damage. If the damaged muscle is perfused myoglobin enters the circulating blood and is filtered by the kidney. Muscle ischemia of 4 hours gives rise to significant myoglobinuria which reaches a maximum approximately 3 hours after the circulation is restored but which persists for as long as 12 hours. Significant myoglobinuria produces myoglobinuric renal failure which may be due to a direct toxic effect of myoglobin to renal vasoconstriction to precipitation of myoglobin in the renal tubules or to a combination of these factors. Most hospitals have sensitive and specific assays for urinary myoglobin. If these are not available dark urine may usually be attributed to myoglobinuria if the benzidine or hemastix tests are positive in the absence of pink serum and microscopic hematuria. If myoglobinuria is suspected one should endeavor to maintain a high urinary output to dilute the effect of myoglobin on the kidney. Because myoglobin is less soluble in acid urine precipitation may be minimized by the maintenance of an alkaline urine through the administration of lactate or bicarbonate. If renal failure ensues prompt institution of dialysis may be required. Whereas myoglobinuric renal failure may complicate any compartmental syndrome it appears to be most common after compartmental syndromes produced by prolonged limb compression in a drug-overdosed patient.
Contractures not infrequently complicate compartment syndromes. l 4 11-15 They appear to result from the shortening of ischemically damaged muscle and from associated nerve damage. Contractures appear to be most common after volar compartmental syndromes of the forearm and deep posterior compartmental syndromes of the leg. In both locations the long flexor muscles of the digits and the nerve supply to the intrinsic muscles are affected. Curiously the muscles of the commonly involved anterior compartment of the leg rarely undergo postischemic contracture.
Contracture from compartmental syndromes is minimized by early compartmental decompression and by appropriate splinting of the limb during the postoperative period. Passive stretching exercises may help maintain muscle length and the range of motion of the joints. If contractures become established some combination of muscle-releasing procedures tendon lengthenings muscle debridement neurolysis tendon transfers and bony procedures may be necessary.
Death has been known to result from compartmental syndromes. In the series reported by Sheridan and Matsen 1 a patient with brittle diabetes died from overwhelming sepsis after delayed surgical decompression. Coupland 16 reported a case of sudden death after surgical decompression and attributed this to the sudden release of a large quantity of acidotic hyperkalemic blood that apparently produced a fatal arrhythmia. When the patient's life is threatened by infection myoglobinuria or other systemic effects of a compartmental syndrome emergency amputation may be life saving.
A standard clinical approach to the patient at risk for a compartmental syndrome is of value in the prevention early detection and treatment of acute compartmental syndromes.
Minimizing morbidity
The following approach is proposed to help minimize the morbidity from compartmental syndromes.
- Prevent compartmental syndromes whenever possible. Effective measures may include prophylactic fasciotomy minimization of soft tissue trauma and ischemia and avoidance of tight circumferential dressings.
- Identify patients at risk. All patients with the potential for significantly increased intracompartmental pressure should be considered to be at risk for a compartmental syndrome. The common causes of increased intracompartmental pressure are listed in Chapter 5. Patients with these conditions require close observation for early evidence of a compartmental syndrome.
- Perform a thorough initial examination and document it well. The initial examination may serve two functions: (a) it helps with the diagnosis or exclusion of a compartmental syndrome at the time this examination is made and (b) it establishes the base line for determining subsequent changes in the patient's condition. For example any deterioration of neuromuscular function after the initial examination would strongly suggest a compartmental syndrome rather than nerve or muscle damage occurring at the time of the initial injury. The patient's chart should reflect the date time and name of the examiner as well as the following information about the compartments at risk: (a) the patient's complaints of pain (b) the strength of the muscles in the compartment (c) the patient's response to passive stretch of the muscles in the compartment (d) the sensation in the distribution of nerves coursing through the compartment and (e) the tenseness of the compartmental envelope.
- Admit patients at significant risk for compartmental syndromes. The frequent examinations that are necessary to permit early diagnosis and treatment are only possible when the patient has been admitted to the hospital. Care should be taken to assure that those observing the patient understand the proper techniques for examination. Uninstructed inexperienced examiners may fail to test specifically for toe extension and fall into the "wiggle your toes" trap (see Chapter 7). They may also be unaware of the important sensory area of the deep peroneal nerve in the first web space and overlook the presence of hypesthesia in that location. When the responsibility of the examination is passed from one individual to another for example at the nurses' change of shift it is very useful for the person coming on duty and the person leaving to perform an examination together; this joint effort eliminates any confusion about the current status of the patient or the technique of the examination.
- Remove circumferential dressings early. The appearance of pain out of proportion to what is expected from the clinical situation deficits in motor or sensory function or pain on passive muscle stretch may well be evidence of a compartmental syndrome. To assure that increased tissue pressure is not resulting from tight circumferential dressings casts should be bivalved (see Chapter 8); one-half of the cast is removed and all soft dressings are split to the skin. Frequently simply splitting the cast does not provide adequate decompression. The consequences of loss of fracture position are insignificant compared with those of a compartmental syndrome.
- Maximize local arterial pressure especially if there is evidence of compartmental ischemia. Systemic hypotension should be treated; local hypotension should be minimized by placing the limb at the level of the heart.
- Utilize tissue pressure measurement particularly if the clinical evaluation is incomplete or confusing. Tissue pressure measurement is a useful adjunct to the clinical evaluation of patients at risk for compartmental syndromes.
- If surgical decompression is indicated promptly and completely open all potentially limiting envelopes. The use of limited skin incisions primary closure of the skin or failure to open all four compartments of the leg may permit the recurrence of compartmental syndromes after surgical decompression.
- Minimize operative debridement. The potential of nerve and muscle for repair or reconstruction after an ischemic insult indicates that only obviously nonviable tissues should be removed at the time of surgical decompression.
- Consider skeletal fixation of unstable fractures associated with compartmental syndromes.
- Delay skin closure until three to five days after surgical decompression. At this time delayed primary closure the application of meshed skin grafts or progressive wound edge approximation may usually be safely instituted. If questionably viable tissue is present closure should be further delayed.
- Minimize contractures by appropriate splinting and range of motion exercises.
- Look for myoglobinuria and other systemic consequences of muscle necrosis. If myoglobinuria is suspected maintain a high urinary output to lessen the nephrotoxic effect.
Syndromes due to exercise
Recurrent leg pain with exercise is a commonly observed symptom. A relatively small number of patients with this symptom have recurrent compartmental syndromes due to intensive use of muscles.
Recurrent compartmental syndromes often produce pain muscle tightness and weakness that require the patient to slow down or cease exercising altogether.
These syndromes may be diagnosed by examination of the patient during and after exercise as well as at rest. Tissue pressure monitoring during a standard exercise test is helpful.
Careful evaluation is required to differentiate this condition from tendinitis shin splints and fatigue fractures.
Click to enlarge
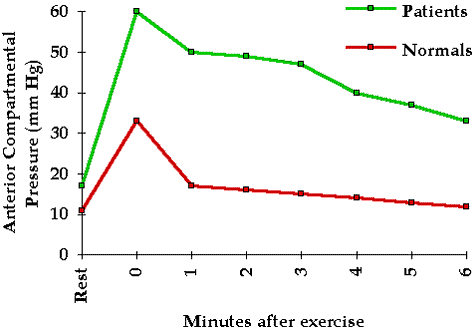
Click to enlarge
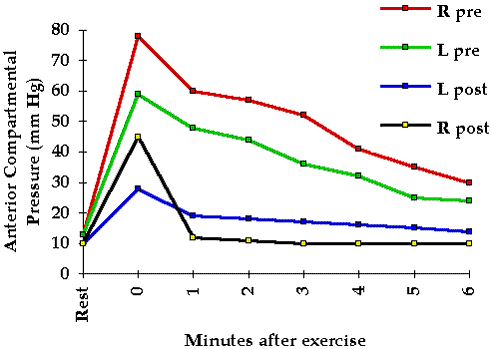
Patients with well-documented recurrent compartmental syndromes due to intensive use of muscles benefit from decompression of the affected compartment.
Intensive muscular work increases muscle volume and thus can lead to increased intracompartmental pressure. Although increased intramuscular pressure from exercise may resolve without producing any symptoms it may also give rise to two varieties of compartmental syndromes: an acute form and a recurrent form. The acute compartmental syndrome from intensive use of muscles is diagnosed and treated as other compartmental syndromes along the lines presented in the foregoing chapters. Recurrent compartmental syndromes from exercise produce a somewhat different clinical picture and thus deserve a separate discussion. The term "recurrent" is preferred over the more familiar term "chronic" because the patient does not have chronic disability but rather is asymptomatic between recurrences. l-6
Pathophysiology
Muscle volume may increase at least 20% with exercise because of both increased capillary filtration and an increased blood content of exercising muscle. 7-9 If the compartmental fascia is sufficiently lax this increase in compartmental content can be accommodated without a significant increase in intracompartmental pressure. However if increased muscle volume with exercise produces an increase in tissue pressure sufficient to interfere with muscle blood flow a compartmental syndrome results. Vigorous muscle contraction alone can increase intramuscular pressure to levels that compromise muscle blood flow. 10 Thus the maintenance of circulation adequate to meet the high metabolic demands of rhythmically exercising muscle requires the rapid recovery of blood flow between contractions. 9 In a recurrent compartmental syndrome tissue pressure remains high between contractions impeding muscle blood flow and producing a relative circulatory insufficiency as long as the vigorous exercise continues.
Diagnosis
Clinically recurrent compartmental syndromes differ from the acute variety in that symptoms are brought on by excessive exercise of the affected compartment and dissipate with a period of rest generally in the order of minutes. Whereas a high degree of exertion is often required to precipitate the symptoms a slower pace of exercise may allow these symptoms to resolve. In many cases symptoms recur predictably with approximately the same amount of exercise.
Recurrent compartmental syndromes of the leg are usually found in athletes and military recruits. The patient typically notes a painful tight sensation in the affected compartment along with weakness of the muscles in that compartment. For example a patient with a recurrent anterior compartmental syndrome of the leg may develop a foot-slap on heel strike due to weakness of the tibialis anterior muscle. Occasionally paresthesias are experienced in the distribution of the nerves running through the affected compartment. Recurrent compartmental syndromes are encountered most frequently in the anterior and lateral compartments of the leg. 5 The deep and superficial posterior compartments of the leg may also be involved.
The physical examination of the nonexercising patient with a recurrent compartmental syndrome is often unremarkable. However Reneman 5 noted fascial hernias in the majority of his patients with this condition. Garfin et al 11 pointed out that these fascial defects tend to occur at the site of emergence of the superficial peroneal nerve. Thus symptoms may arise from the compartmental syndrome from herniation of muscle through the defect or from local compression of the nerve.
Because this syndrome is produced by exercise it is most useful to examine the compartment during and after vigorous exertion of the muscles in the compartment. The compartment may be most conveniently exercised by asking the patient to repeatedly contract the compartmental muscles against manual resistance until characteristic symptoms are produced. At this point the compartment may be palpated for tenseness and the muscles examined for weakness. When involvement is unilateral the opposite side is used for comparison. The patient may also be asked to perform exactly the exercise that causes his symptoms with the physician running or biking at his side. This type of "on the scene" evaluation gives the physician the most accurate idea of what is occurring in the patient's extremities. Pain that occurs with the first few steps but that can be "run through cannot be attributed to a recurrent compartmental syndrome. Pain that comes on after a more or less predictable amount of exercise and that requires the patient to slow his pace or stop exercising is much more typical, particularly if associated with a tight compartment and weakness of the intracompartmental muscles.
Reneman 5 provided good evidence that increased tissue pressure is important in recurrent compartmental syndromes. With the use of an injection technique, he measured tissue pressures in the anterior compartment of the leg before exercise and at zero, three, and six minutes after a standard exercise test (repeated dorsiflexion of the foot against resistance). This test was carried out in normal volunteers and in a group of male patients in whom the need for surgical decompression had been determined on clinical grounds. Resting pressures were only slightly elevated in the patients requiring surgical decompression. However, the tissue pressure six minutes after exercise was significantly increased in all 34 of these patients.
We have used the continuous infusion technique (see Chapter 2) in a similar exercise test to evaluate patients for recurrent compartmental syndromes. In this application an 18-gauge catheter and an infusion rate of 0.1 cc per hour provide a better dynamic response than the smaller catheter and slower infusion rate used in monitoring limbs at risk for acute compartmental syndromes. Use of the infusion technique provides continuous pressure monitoring during and immediately after exercise. With the catheter in the muscle of the compartment, base-line readings are obtained. The compartmental muscles are then contracted against resistance at a rate of one per second for three minutes. Particular notice is taken if the patient's symptoms are reproduced during the exercise test. In the examination of the anterior compartment of the leg, resistance to foot dorsiflexion may be applied manually or with the use of a hinged footboard connected through a pulley to a 6-kg weight.
We studied seven anterior compartments of the leg in five patients believed to have recurrent compartmental syndromes because of their clinical findings. We also studied a control group consisting of six male and six female volunteers (age range- 12 to 61 years; average age, 28 years). The results are quite interesting. In our patient group, resting anterior compartment pressure averaged 16+2 mm Hg compared with 11+2 mm Hg in our control group (mean +SD). The postexercise pressure curve in the patient group deviated dramatically from that of the control group. For the patients, the postexercise pressures were higher and did not return to pre-exercise levels within six minutes. These results are identical to those of Reneman. 5
Differential diagnosis
The common diagnoses requiring differentiation from recurrent compartmental syndromes include tendinitis fatigue fractures and the poorly understood entity known as shin splints. These conditions are probably more common causes of exercise-related leg pain than are recurrent compartmental syndromes. Although they may produce leg symptoms similar to those of recurrent compartmental syndromes these conditions are not accompanied by indications of increased intracompartmental pressure. In addition whereas many patients can run through symptoms due to these conditions such is not the case with compartmental syndromes.
Symptoms of tendinitis usually persist after the exercise has been stopped; pain is often reproduced by passively stretching the affected tendon. In fatigue fractures a sharply defined area of bone tenderness usually extends Mom one side of the bone to the other. Radiographic evidence of periosteal new bone formation may be present in long-standing cases. Bone scans frequently indicate locally increased bone turnover. In shin splints pain is usually located just behind the medial tibial crest often at the junction of the middle and distal thirds of the tibia. The area of tenderness is often 10 cm or more in length. While roentgenograms remain normal the bone scan may show increased bone turnover along the area of tenderness. In our experience patients with shin splints do not demonstrate increased tissue pressure at rest or after exercise. Therefore we cannot recommend surgical decompression of the deep posterior compartment in the treatment of this condition as suggested by Puranen. 12
Treatment
Many patients with recurrent compartmental syndromes due to intensive use of muscles are relieved to gain an understanding of their condition and are willing to modify their exercise program to avoid the resulting symptoms. Some serious athletes however are unable to modify their exercise program and request surgical decompression.
In recurrent compartmental syndromes due to intensive use of muscles the surgical procedure is quite different from that used for treating acute compartmental syndromes. First the procedure is not an emergency. Second one compartment can usually be clearly identified as being responsible for the patient's symptoms. Third postischemic swelling is not anticipated after the operative procedure; thus subcutaneous fasciotomy is appropriate. The fascial incision is made through two small skin incisions and runs the entire length of the compartment leaving no fascial bridges. Care is required to avoid injuring the branches of the superficial peroneal nerve in decompressing the anterior compartment of the leg as pointed out by Garfin et al. 11 At the end of the procedure the skin is closed with a cosmetic suture. The patient is warned that the extremity may swell with dependency for a few days up to a few weeks after the procedure. A progressive exercise program is instituted one week after surgery.
To date we have operated on five anterior compartments of the leg in four patients. These have included a runner a race walker an ice skater and a professional soccer referee. All had significant improvement after their surgical procedure and returned to their activities. Reneman 5 6 also noted excellent results from his treatment of patients with this condition. Thirty-six of 40 patients who submitted to surgery were able to resume physical activities that had been prohibited by symptoms before surgery. One patient did not experience improvement and three were lost to follow-up.
The following case report presents an instructive example of a recurrent compartmental syndrome due to intensive use of muscles:
A 32-year-old white male world class race walker had a 15-year history of painful tightness in both anterior compartments during exercise. His symptoms would typically appear in the first three or four miles of race walking at a competitive speed although they could be avoided if he walked at a somewhat slower pace. The pain was accompanied by weakness of foot dorsiflexion noted as a foot-slap on heel strike. The patient also observed a vague numbness over the dorsum of his foot after the onset of pain. Although he was able to complete longer races and marathons his speed was retarded by his symptoms.
Routine physical examination was unremarkable. No fascial hernias were detected. Upon repeated dorsiflexion of his foot against resistance his anterior compartments became tense and his symptoms were reproduced. Formal exercise tests were conducted while anterior compartmental pressures were monitored using the continuous infusion technique. Resting anterior compartment pressures measured 15 mm Hg on the left and 14 mm Hg on the right. Postexercise pressures were markedly elevated and showed a retarded return toward the pre-exercise level.
Subcutaneous fasciotomies of both anterior compartments were performed. Six weeks after operation the patient was asymptomatic. A repeat pressure test during exercise at this time revealed a normal response. The patient returned to full training and competition. He placed in the top five in the Pan American games six months after surgery and at this writing is a strong candidate for the United States Olympic race walking team.
Challenges in diagnosis and treatment
Although the diagnosis and treatment of some compartmental syndromes may be straightforward other cases can be quite challenging. In some instances the physician is pressed to make an early diagnosis of a compartmental syndrome so that prompt surgical decompression can be accomplished. In other situations the physician must exclude the diagnosis of a compartmental syndrome to avoid performing unnecessary surgery.
Seven cases
Having reviewed most of the available information on compartmental syndromes the reader may now find it interesting to study some cases that demonstrate problems in the diagnosis and management of this condition. Seven such cases are presented below. These cases have been arranged to challenge the reader to apply his knowledge in selecting the appropriate laboratory evaluation and treatment without being biased by what actually occurred. Thus the history and clinical evaluation are presented separately from the subsequent course.
In the first three cases earlier diagnosis and treatment as well as a better end result might have been possible had the physician originally treating the patient been more familiar with compartmental syndromes. The last four cases demonstrate that careful clinical evaluation and adjunctive diagnostic tests can help resolve some very challenging diagnostic problems.
Case 1
History and clinical evaluation
A 47-year-old male truck driver was in good health until he noticed the acute onset of anterior chest pain radiating down both arms while he performed push-ups. He came to the hospital in acute distress where a dissecting aneurysm of the ascending aorta was diagnosed. An emergency surgical repair was performed. This procedure was difficult and required 5 hours and 12 minutes of cardiopulmonary bypass using the right femoral artery. Cannulation of this artery in a retrograde manner produced a relative occlusion of the femoral artery.
After operation the patient was in serious condition in the intensive care unit. The neuromuscular function of his right leg was not checked until a consulting physician examined him approximately 14 hours after the conclusion of the original operation. This examination revealed a tense right leg from the knee to the ankle. The patient was unable to move his toes and had no sensation in his foot. There was pain on passive stretch in both the anterior and deep posterior compartments. His distal pulses were intact.
Laboratory evaluation treatment and result
The presence of a tense leg with severe neuromuscular deficits was deemed sufficient to establish the diagnosis of a compartmental syndrome and to justify immediate surgical decompression; no additional time was taken for diagnostic procedures. A four-compartment parafibular decompression was performed. The contents of all compartments bulged markedly. The muscle of the anterior compartment was quite dusky. This patient's subsequent clinical course was complicated by myoglobinuric renal failure that responded to hemodialysis. His wound was treated open with daily dressing changes for 13 days at which time he was taken to the operating room for inspection of the wound and skin grafting. The anterior compartment appeared to be pale and the extensor digitorum longus muscle was necrotic and required excision. Minimal debridement of the tibialis anterior and extensor hallucis longus muscles was performed. The rest of the leg muscles appeared healthy. A meshed split-thickness graft was applied. Eighty-five percent of the graft took primarily. The remainder of the wound was allowed to heal by granulation and epithelialization.
One year after surgery the patient had grade four strength of the muscles of the lateral superficial posterior and deep posterior compartments. The tibialis anterior muscles which had apparently been functionless for over six months had recovered grade three strength and the patient no longer needed a drop foot brace. The patient's heart aortic renal and cerebral function were all normal.
Comment
This case was made difficult by the patient's critical condition and by the intensive medical and surgical treatment required to save his life. In retrospect prophylactic fasciotomy may have been indicated in view of the massive postischemic swelling expected after the release of prolonged occlusion of the femoral artery. The muscle of the anterior compartment obviously sustained a double ischemic insult first from the arterial occlusion and then from the compartmental syndrome. It is ironic that although the function of his anterior compartment seemed insignificant while the patient was critically ill the loss of this function is now his major disability. It is also instructive to note the delayed functional return of sufficient anterior compartmental function to make him brace free.
Case 2
History and clinical evaluation
A l6 year-old boy had surgical correction of a 20-degree valgus deformity of the right tibia. The osteotomy was performed just distal to the tibial tubercle along with a proximal fibular osteotomy. On awaking from anesthesia the patient was unable to extend his toes or dorsiflex his foot. Hypesthesia was present in the distribution of the deep and superficial peroneal nerves. Twenty-four hours after operation the patient complained of increasing pain in the leg which responded incompletely to removal of the circumferential dressings. A consulting physician examined the patient two days later and noted anesthesia in the distribution of the deep peroneal nerve. Strength of toe flexion was four out of five; strength of toe extension was zero out of five. The leg was moderately tight on palpation particularly in the proximal aspect of the anterior compartment.
Laboratory evaluation treatment and result
This patient had at least two causes for the neuromuscular deficits: a peroneal nerve injury at the time of surgery and an anterior compartmental syndrome. It was possible that these lesions coexisted. To help in determining the need for surgical decompression tissue pressure was measured at the point of maximum tenseness in the anterior compartment: a value of 50 mm Hg was obtained.
A four-compartment parafibular decompression was performed; the contents of the anterior compartment were necrotic and complete debridement was subsequently required. The wound was closed eventually with a meshed split-thickness graft. The patient is currently using a drop foot brace two months after surgery.
Comment
This case was made difficult by the two possible etiologies of loss of anterior compartmental function: a compartmental syndrome and a peroneal nerve palsy. A compartmental syndrome is differentiated from a nerve palsy by the presence of inappropriate pain and by the demonstration of increased tissue pressure. Thus earlier evaluation of the tenseness of the anterior compartment either by palpation or by pressure measurement might have prevented the delayed diagnosis of a compartmental syndrome. Decompression two days after the onset of a compartmental syndrome cannot be expected to restore normal function. Prophylactic fasciotomy at the time of the osteotomy may have been effective in preventing the anterior compartmental syndrome.
Case 4
History and clinical evaluation
An l8-year-old man sustained an anterior dislocation of his left knee while playing football. After reduction of the knee examination revealed a swollen proximal leg absent active extension of the toes hypesthesia in the distributions of the deep and superficial peroneal nerves and a diminished dorsalis pedis pulse.
Laboratory evaluation treatment and result
An arteriogram revealed a small intimal tear near the origin of the anterior tibial artery. Stimulation of the peroneal nerve at the fibular neck produced strong extension of the toes. Anterior compartment pressure measurements reached a maximum of 15 mm Hg. These data indicated that the paralysis was not due to compartmental ischemia but rather to an injury of the peroneal nerve proximal to the fibular neck. The arterial lesion was not treated. Peroneal nerve function gradually returned.
Comment
This case presented a classical differential diagnosis: anterior compartmental syndrome of the leg versus peroneal nerve palsy versus occlusion of the anterior tibial artery. The pressure measurements were helpful in excluding a compartmental syndrome. The results of nerve stimulation demonstrated that the paralysis of the compartment was not due to ischemia of the compartmental contents. Thus peroneal nerve palsy became the most likely diagnosis.
Case 5
History and clinical evaluation
A 34-year-old woman lay on her left side for 24 hours after a barbiturate overdosage. After awaking she noticed an inability to dorsiflex her foot or extend her toes. The antero-lateral leg was swollen but the compartments did not appear clinically tense.
Laboratory evaluation treatment and result
Peroneal nerve stimulation distal to the fibular neck elicited normal foot dorsiflexion and toe extension. Anterior compartment pressures reached a maximum of 22 mm Hg. Subsequent formal nerve conduction velocity measurement and electromyography confirmed the diagnosis of common peroneal nerve palsy from direct pressure. There was no subsequent evidence of compartmental or crush syndromes. Myoglobinuria was absent. Neurological function of the leg completely returned.
Comment
Drug overdosage with prolonged recumbency is a classical etiology of compartmental syndromes. In this case however the lack of pain and compartmental tenseness as well as the results of the adjunctive diagnostic tests ruled out the diagnosis of a compartmental syndrome and helped prevent an unnecessary surgical decompression.
Case 6
History and clinical evaluation
A 60-year-old female pedestrian was hit by an automobile traveling approximately 70 mph. She sustained multiple trauma including a depressed skull fracture a pelvic fracture an intertrochanteric fracture of the right femur and a spiral fracture of the right tibia with significant soft tissue injury. This women was obviously at high risk for a compartmental syndrome in the right leg but routine examination was impossible because she was comatose from her head injury.
Laboratory evaluation treatment and result
Intermittent stimulation of the right peroneal nerve provided assurance that her local neuromuscular status was intact over the first 72 hours including the time when intracompartmental pressure rose to its maximum of 45 mm Hg. The patient continued to recover from her injuries and as of two months after her accident had no neurologic sequelae in her right lower extremity.
Comment
The Babinski sign and withdrawal reflexes may be of use in determining the functional status of the leg compartments in a comatose patient. In this situation further diagnostic assistance may be derived from tissue pressure monitoring and direct nerve stimulation.
Case 7
History and clinical evaluation
A l3-year-old female cross-country runner experienced pain in the anterior compartment of the right leg each time she ran. Initially she could "run through" her symptoms. For the three-month period before evaluation however her symptoms were sufficiently severe to prevent her from running at all. She had not noticed weakness or sensory changes in her leg or foot with exercise. Examination at rest was normal except for slight tenderness in the distal anterior compartment.
Laboratory evaluation treatment and result
Repeated dorsiflexion of the foot against resistance reproduced her symptoms but was not associated with increased tissue pressure either by palpation or by pressure measurement. On this basis the diagnosis of a recurrent compartmental syndrome due to intensive use of muscles was rejected. The patient subsequently responded to treatment for anterior tibial tendinitis.
Comment
Recurrent compartmental syndromes are a relatively uncommon cause of exercise-related pain. The diagnosis should be well established before surgical treatment is contemplated.
1. About compartmental syndromes
- Sheridan GW Matsen FA: Fasciotomy in the treatment of the acute compartment syndrome. J Bone Jt Surg (Am) 58:112115 1976
- Parkes AR: Traumatic ischaemia of peripheral nerves with some observations on Volkmann's ischemic contracture. Br J Surg 32:403-414 1945
- Thomson SA Mahoney LJ: Volkmann's ischaemic contracture and its relationship to fracture of the femur. J Bone Jt Surg (Br) 33:336-347 1951
- McQuillan WM Nolan B: Ischaemia complicating injury. A report of thirty-seven cases. J Bone Jt Surg (Br) 50:482-492 1968
- Holden CEA: Traumatic tension ischaemia in muscles. Injury 5:223-227 1973
- Spinner MA Mache A Silver L et al: Impending ischemic contracture of the hand. Plast Reconstr Surg 50:341-349 1972
- Kirby NG: Exercise ischaemia in the fascial compartment of soleus. Report of a case. J Bone Jt Surg (Br) 52:738-745 1970
- Tompkins DG: Exercise myopathy of the extensor carpi ulnaris muscle. Report of a case. J Bone Jt Surg (Am) 59:407-408 1977
- Bradley EL: The anterior tibial compartment syndrome. Surg Gynecol Obst 136:289-297 1973
- Reneman RS: The Anterior and the Lateral Compartment Syndrome of the Leg. The Hague Mouton 1968 p 176
- Reszel PA Janes JM Spittell JA: Ischemic necrosis of the peroneal musculature a lateral compartment syndrome: report of a case. Mayo Clin Proc 38:130-136 1963
- Weitz EM Carson G: The anterior tibial compartment syndrome in a twenty month old infant. A complication of the use of a bow leg brace. Bull Hosp Jt Dis 30:16-20 1969
- Zweifach SS Hargens AR Evans KL et al: Skeletal-muscle injury in pressurized compartments associated with hemorrhagic hypotension. Microvasc Res 17 (Part 2):S125 1979
- Fowler PJ Willis RB: Vascular compartment syndromes. Can J Surg 18:157-161 1975
- Carter AB Richards RL Zachary RB: The anterior tibial syndrome. Lancet II:928-934 1949
- Mans on I W: Post-partum eclampsia complicated by anterior tibi al syndrome. Br Med J 2:1117-1118 1964
- Sweeney HE O'Brien GF: Bilateral anterior tibial syndrome in association with the nephrotic syndrome: report of a case. Arch Intern Med 116:487-490 1965
- Puranen J: The medial tibial syndrome. Exercise ischaemia in the medial fascial compartment of the leg. J Bone Jt Surg (Br) 56:712715 1974
- Klock JC Sexton MJ: Rhabdomyolysis and acute myoglobinuric renal failure following heroin use. Calif Med 119:5-8 1973
- Gaspard DJ Cohen JL Gaspar MR: Decompression dermotomy. A limb salvage adjunct. JAMA (J Am Med Assoc) 220:831-833 1972
- Matsen FA: Compartmental syndrome. A unified concept. Clin Orthop Relat Res 113:8-14 1975.
2. Tissue pressure and its management
- Wiederhielm CA: The capillaries veins and Iymphatics in Ruch TC Patton HD (eds): Physiology and Biophysics. Circulation Respiration and Fluid Balance vol 2. Philadelphia Saunders 1974 p 136
- Hargens AR Akeson WH Mubarak SJ et al: Fluid balance within the canine anterolateral compartment and its relationship to compartment syndrome. J Bone Jt Surg (Am) 60:499-505 1978
- Ashton H: The effect of increased tissue pressure on blood flow. Clin Orthop Relat Res 113:15-26 1975
- Wiederhielm CA: The interstitial space in Fung YC Perrone N. Anliker M (eds): Biomechanics: Its Foundations and Objectives. New Jersey Prentice-Hall 1970 p 273
- Guyton AC Granger HJ Taylor AE: Interstitial fluid pressure. Physiol Rev 51:527-563 1971
- Ladegaard-Pedersen HJ: Measurement of the interstitial pressure in subcutaneous tissue in dogs. Circ Res 26:765770 1970
- Prather JW Bowes DN Warrell DA et al: Comparison of capsule and wick techniques for measurement of interstitial fluid pressure. J Appl Physiol 31:942-945 1971
- Stromberg DD Wiederhielm CA: Effects of oncotic gradients and enzymes on negative pressures in implanted capsules. Am J Physiol 219:928-932 1970
- Gregg DE Eckstein RW: Measurements of intramyocardial pressure. Am J Physiol 132:781-790 1941
- Johnson JR Di Palma JR: Intramyocardial pressure and its relation to aortic blood pressure. Am J Physiol 125:234-243 1939
- Ryder HW Molle WE Ferris EB: The influence of the collapsibility of veins on venous pressure including a new procedure for measuring tissue pressure. J Clin Invest 23:334-341 1943
- Kjellmer I: An indirect method for estimating tissue pressure with special reference to tissue pressure in muscle during exercise. Acta Physiol Scand 62:31-40 1964
- Henderson Y. Oughterson AW Greenburg LA: Muscle tonus intramuscular pressure and the venopressor mechanism. Am J Physiol 114:261-268 1936
- Burch GE Sodeman WA: The estimation of the subcutaneous tissue pressure by a direct method. J Clin Invest 16:845-850 1937
- Wells HS Youmans JB Miller DG: Tissue pressure {intracutaneous suboutaneous and intramuscular) as related to venous pressure capillary filtration and other factors. J Clin Invest 17:489-499 1938
- Reneman RS: The anterior and the lateral compartmental syndrome of the leg due to intensive use of muscles. Clin Orthop Relat Res 113:69-80 1975
- Whitesides TE Harada H. Morimoto K: Compartment syndromes and the role of fasciotomy its parameters and techniques in Instructional Course Lectures The American Academy of Orthopedic Surgeons vol 26. St Louis Mosby 1977 p 179
- Hargens AR Mubarak SJ Owen CA et al: Interstitial fluid pressure in muscle and compartment syndrome in man. Microvasc Res 14:1- 10 1977
- Clayton JM Hayes AC Barnes RW: Tissue pressure and perfusion in the compartment syndrome. J Surg Res 22:333-339 1977
- Scholander PF Hargens AR Miller SL: Negative pressure in the interstitial fluid of animals. Science 161:321328 1968
- Snashall PD Boother FA: Interstitial gel swelling pressure in human subcutaneous tissue measure with a cotton wick. Clin Sci Mol Med 46:241-251 1974
- Mubarak SJ Hargens AR Owen CA et al: The wick catheter technique for measurement of intramuscular pressure. A new research and clinical tool. J Bone Jt Surg (Am) 58:1016- 1021 1976
- Zeluff GR: Absorbable versus nonabsorbable wick material in eompartment pressure monitoring. Paper presented at the Western Orthopedic Association Meeting Seattle Washington 1-5 October 1978
- Matsen FA Krugmire RB King RV: Increased tissue pressure and its effect on muscle oxygenation in level and elevated human limbs. Nicholas Andry Award. Clin Orthop Relat Res 144:318-327 1979
- Matsen FA Mayo KA Sheridan GW et al: Monitoring of intramuscular pressure. Surgery (St. Louis) 79:702-709 1976
- Matsen FA Winquist RA Krugmire RB: Diagnosis and management of compartmental syndromes. J Bone Jt Surg (Am) 62:286-291 1980
- Katz MA: Validity of interstitial fluid hydrostatic pressure measurement in hollow porous polyethylene capsules. Microvasc Res 16:316-326 1978
- McMaster PD: The pressure and interstitial resistance prevailing in the normal and edematous skin of animals and man. J Exp Med 84:473-494 1946
- Swann HG Montgomery AV Davis JC et al: A method for rapid measurement of intrarenal and other tissue pressures. J Exp Med 92:625-636 1950
- Meyer F. Holland G: Die Messung des Druckes in Geweben. Arch Exp Pathol Pharmakol 168:580-602 1932
- Landerer A: Die Gewebspannung in ihrem Einfluss auf die ortliche Blut und Lymphbewegung. Leipsig FCW Vogel 1884
- Matsen FA Wyss CR Krugmire RB et al: The effects of limb elevation and dependency on local arteriovenous gradients in normal human limbs with particular reference to limbs with increased tissue pressure. Clin Orthop Relat Res 150 July-Aug 1980
- Dahn I Lassen NA Westling H: Blood flow in human muscles during external pressure or venous stasis. Clin Sci 32:467-473 1967
- Ashton H: Effect of inflatable plastic splints on blood flow. Br Med J 4:1427-1430 1966
- Thomson AE Doupe J: Some factors affecting the auscultatory measurement of arterial blood pressures. Can J Res Sect E Med Sci 27:72-80 1949/1950
3. Pathophysiology
- Ashton H: The effect of increased tissue pressure on blood flow. Clin Orthop Relat Res 113:15-26 1975
- Rorabeck CH Clarke KM: The pathophysiology of the anterior tibial compartment syndrome: an experimental investigation. J Trauma 18:299-304 1978
- Rorabeck CH MacNab I: The pathophysiology of the anterior tibial compartmental syndrome. Clin Orthop Relat Res 113:52-57 1975
- Dahn I Lassen NA Pestling H: Blood flow in human muscles during external pressure or venous stasis. Clin Sci 32:467-473 1967
- Sheridan GW Matsen FA Krugmire RB: Further investigations on the pathophysiology of the compartmental syndrome. Clin Orthop Relat Res 123:266-270 1977
- Matsen FA King RV Krugmire RB et al: Physiological effects of increased tissue pressure. Int Orthop (SICOT) 3:237-244 1979
- Brantigan JW: Catheters for continuous in vivo blood and tissue gas monitoring. Crit Care Med 4:239-244 1976
- Matsen FA Krugmire RB King RV: Increased tissue pressure and its effects on muscle oxygenation in level and elevated human limbs. Nicholas Andry Award. Clin Orthop Relat Res 144:311-320 1979
- Nicholas GG Miller SH: The anterior tibial compartment syndrome: tissue gas tension measurement. J Surg Res 24:334-338 1978
- Matsen FA Krugmire RB King RV: Physiological effects of increased tissue pressure and of elevation. Transactions of the 25th Annual Meeting Orthop Res Soc 4:15 1979
- Hargens AR Romine JS Sipe JC et al: Peripheral nerve-conduction block by high muscle-compartment pressure. J Bone Jt Surg (Am) 61:192-200 1979
- Matsen FA Mayo KA Krugmire RB et al: A model compartmental syndrome in man with particular reference to the quantification of nerve function. J Bone Jt Surg (Am) 59:648-653 1977
- Matsen FA King RV Wyss CR et al: Effect of acute hemorrhage and arterial ligation on the tolerance of muscle for increased tissue pressure. Transactions of the 26th Annual Meeting Orthop Res Soc 5 1980
- Benjamin A: The relief of traumatic arterial spasm in threatened Volkmann's ischaemic contracture. J Bone Jt Surg (Br) 39:711-713 1957
- Eaton RG Green WT: Epimysiotomy and fasciotomy in the treatment of Volkmann's ischemic contracture. Orthop Clin North Am 3:175-186 1972
- Foisie PS: Volkmann's ischemic contracture. An analysis of its proximate mechanism. N Engl J Med 226:671679 1942
- Gardner RC: Impending Volkmann's contracture following minor trauma to the palm of the hand. A theory of pathogenesis. Clin Orthop Relat Res 72:261-264 1970
- Matsen FA Krugmire RB: Compartmental syndromes. Surg Gynecol Obst 147:943-949 1978
- Matsen FA Clawson DK: The deep posterior compartmental syndrome of the leg. J Bone Jt Surg (Am) 57:3439 1975
- Mubarak SJ Owen CA: Compartmental syndrome and its relation to the crush syndrome: a spectrum of disease. A review of 11 cases of prolonged limb compression. Clin Orthop Relat Res 113:81-89 1975
- Burton AC: Relation of structure to function of the tissues of the wall of blood vessels. Physiol Rev 34:619642 1954
- Burton AC: On the physical equilibrium of small blood vessels. Am J Physiol 164:319-329 1951
- Henriksen O: Orthostatic changes of blood flow in suboutaneous tissue in patients with arterial insufficiency of the legs. Scand J Clin Lab Invest 34:103- 109 1974
- Hargens AR Akeson WH Mubarak SJ et al: Fluid balance within the canine anterolateral compartment and its relationship to compartment syndromes. J Bone Jt Surg (Am) 60:499-505 1978
- Hargens AR Mubarak SJ Owen CA et al: Interstitial fluid pressure in muscle and compartment syndrome in man. Microvasc Res 14:1 - 10 1977
- Duffield FA Harris I: Increase of pressure in veins to level of arterial pressure caused by constricting the limb in which the venous pressure is recorded. J Physiol (Lond) 81:283-285 1934
- Matsen FA Wyss CR Krugmire RB et al: The effects of limb elevation and dependency on local arteriovenous gradients in normal human limbs with particular reference to limbs with increased tissue pressure. Clin Orthop Relat Res 150 July-Aug 1980
- Ryder HW Molle WE Ferris EB: The influence of the collapsibility of veins on venous pressure including a new procedure for measuring tissue pressure. J Clin Invest 23:334-341 1943
- Kjellmer I: An indirect method for estimating tissue pressure with special reference to tissue pressure in muscle during exercise. Acta Physiol Scand 62:31-40 1964
- Reneman RS: The anterior and the lateral compartmental syndrome of the leg due to intensive use of muscles. Clin Orthop Relat Res 113:69-80 1975
- Matsen FA: Compartmental syndrome. A unified concept. Clin Orthop Relat Res 113:8-14 1975
- Feigl EO: Physics of the cardiovascular system in Ruch TC Patton HD (eds): Physiology and Biophysics. Circulation Respiration and Fluid Balance vol 2. Philadelphia Saunders 1974 p 15
- Feigl EO: The arterial system in Ruch TC Patton HD (eds): Physiology and Biophysics. Circulation Respiration and Fluid Balance vol 2. Philadelphia Saunders 1974 p 121
4. Pressure tolerance
- Sheridan GW Matsen FA Krugmire RB: Further investigations on the pathophysiology of the compartmental syndrome. Clin Orthop Relat Res 123:266-270 1977
- Matsen FA King RV Krugmire RB et al: Physiological effects of increased tissue pressure. Int Orthop (SICOT) 3:237-244 1979
- Rorabeck CH Clarke KM: The pathophysiology of the anterior tibial compartment syndrome: an experimental investigation. J Trauma 18:299304 1978
- Hargens AR Romine JS Sipe JC et al: Peripheral nerve-conduction block by high muscle-compartment pressure. J Bone Jt Surg (Am) 61:192-200 1979
- Matsen FA Mayo KA Krugmire RB et al: A model compartmental syndrome in man with particular reference to the quantification of nerve function. J Bone Jt Surg (Am) 59:648-653 1977
- Hargens AR Mubarak SJ Akeson WH et al: Critical pressure and time relationships in compartment syndromes. International Research Society for Orthopedics and 7. Traumatology (SIROT) First Meeting Program and Abstracts 15-20 October 1978 Kyoto Japan p 21
- Hargens AR Evans KL Hagan PL et al: Skeletal muscle necrosis in pressurized compartments as assessed by technetium-99m stannous pyrophosphate. Transactions of the 24th Annual Meeting Orthop Res Soc 3:48 1978
- Lundborg G: Ischemic nerve injury. Experimental studies on intraneural microvascular pathophysiology and nerve function in a limb subjected to temporary circulatory arrest. Scand J Plast Reconstr Surg Suppl 6:3-113 1970
- Sanderson RA Foley RK McIvor GWD et al: Histological response of skeletal muscle to ischemia. Clin Orthop Relat Res 113t27-35 1975 Vracko R. Benditt EP: Basal lamina: the scaffold for orderly cell replacement. Observations on regeneration of injured skeletal muscle fibers and capillaries. J Cell Biol 55:406-419 1972
- Matsen FA Wyss CR King RV et al: Effect of acute hemorrhage on transcutaneous subcutaneous intramuscular and arterial oxygen tensions. Pediatrics 65 May 1980
- Zweifach SS Hargens AR Evans KL et al: Skeletal-muscle injury in pressurized compartments associated with hemorrhagic hypotension. Microvasc Res 17(Part 2):S125 1979
- Matsen FA Wyss CR Krugmire RB et al: The effects of limb elevation and dependency on local arteriovenous gradients in normal human limbs with particular reference to limbs with increased tissue pressure. Clin Orthop Relat Res 150 July-Aug 1980
- Feigl EO: Physics of the cardiovascular system in Ruch TC Patton HD (eds): Physiology and Biophysics. Circulation Respiration and Fluid Balance vol 2. Philadelphia Saunders 1974 p 11
- Matsen FA King RV Krugmire RB et al: Physiological effects of increased tissue pressure. Int Orthop (SICOT) 3:237-244 1979
Supplemental reading dealing with the effect of is
Bowden REM Gutmann E: The fate of voluntary muscle after vascular injury in man. J Bone Jt Surg (Br) 31:356-368 1949
Clark MW D'Ambrosia RD Roberts JM: Equinus contracture following Bryant's traction. Orthopedics 1:311-312 1978
Dahlback L-O: Effects of temporary tourniquet ischemia on striated musele fibers and motor end-plates. Scand J Plast Reconstr Surg Suppl 7:7-91 1970
Hargens AR Romine JS Sipe JC et al: Peripheral nerve-conduction block by high muscle-compartment pressure. J Bone Jt Surg (Am) 61:192-200 1979
Harman JW Gwinn RP: The recovery of skeletal muscle fibers from acute ischemia as determined by histologic and chemical methods. Am J Pathol 25:741 -755 1949
Holmes W. Highet WB Seddon HJ: Ischaemic nerve lesions occurring in Volkmann's contracture. Br J Surg 32:259-275 1944
Lundborg G: Ischemic nerve injury. Experimental studies on intraneural microvascular pathophysiology and nerve function in a limb subjected to temporary circulatory arrest. Scand J Plast Reconstr Surg Suppl 6:3-113 1970
Malan E Tattoni G: Physio- and anatomo-pathology of acute ischemia of the extremities. J Cardiovasc Surg 17:212-225 1963
Miller HH Welch CS: Quantitative studies on the time factor in arterial inJuries. Ann Surg 130:428-438 1949
Montagnani CA Simeone FA: Observations on the liberation and elimination of myohemoglobin and of hemoglobin after release of muscle ischemia. Surgery 34:169-185 1953
Parkes AR: Traumatic ischemia of peripheral nerves with some observations on Volkmann's ischaemic contracture. Br J Surg 32:403-414 1945
Schreiber SN Liebowitz MR Bernstein LH: Limb compression and renal impairment (crush syndrome) following narcotic and sedative overdose. J Bone Jt Surg (Am) 54:1683-1692 1972
Scully RE Shannon JM Dickersin GR: Factors involved in recovery from experimental skeletal muscle ischemia produced in dogs. Am J Pathol 39:721737 1961
Speckman EJ Caspers H. Bingmann D: Actions of hypoxia and hypercapnia on single mammalian neurons in Bicher HI Bruley DF (eds): Oxygen Transport to Tissue Instrumentation Methods and Physiology vol 37. New York Plenum 1973 p 245
Spinner MA Mache A Silver L et al: Impending ischemic contracture of the hand. Plast Reconstr Surg 50:341-349 1972
Whitesides TE Harada H. Morimoto K: Compartment syndromes and the role of fasciotomy its parameters and technique in Instructional Course Lectures The American Academy of Orthopedic Surgeons vol 26. St Louis Mosby 1977 p 179
Wright EB: A comparative study of the effects of oxygen lack on peripheral nerve. Am J Physiol 147:78-88 1946
5. Etiologies
- Owen CA Woody PR Mubarak SJ et al: Gluteal compartment syndromes: a report of three cases and management utilizing the wick catheter. Clin Orthop Relat Res 132:57-60 1978
- Patman RD Thompson JE: Fasciotomy in peripheral vascular surgery. Arch Surg 101:663-670 1970
- Gaspard DJ Cohen JL Gaspar MR: Decompression dermotomy. A limb salvage adjunct. JAMA (J Am Med Assoc) 220:831-833 1972
- Gaspard DJ Kohl RD: Compartmental syndromes in which the skin is the limiting boundary. Clin Orthop Relat Res 113:65-68 1975
- Mann RJ Wallquist JM: Early decompression fasciotomy in the treatment of high-voltage electrical burns of the extremities. South Med J 68:1103-1108 1975
- Patman RD: Compartmental syndromes in peripheral vascular surgery. Clin Orthop Relat Res 113:103-110 1975
- Justis DL Law EJ MacMillan BG: Tibial compartment syndromes in burn patients. A report of four cases. Arch Surg 111:1004 - 1008 1976
- Eaton RG Green WT: Epimysiotomy and fasciotomy in the treatment of Volkmann's ischemic contracture. Orthop Clin North Am 3:175-186 1972
- Eaton RG Green WT: Volkmann's ischemia. A volar compartment syndrome of the forearm. Clin Orthop Relat Res 113:58-64 1975
- Volkmann R von: Ischaemic muscle paralyses and contractures. Bick EM (trans). Clin Orthop Relat Res 50:5-6 1967
- Whitesides TE Harada H. Morimoto K: Compartmental syndromes and the role of fasciotomy its parameters and techniques in Instructional Course Lectures The American Academy of Orthopedic Surgeons vol 26. St Louis Mosby 1977 p 179
- Hargens AR Akeson WH Mubarak SJ et al: Fluid balance within the canine anterolateral compartment and its relationship to compartment syndromes. J Bone Jt Surg (Aml 60:499-505 1978
- Sheridan GW Matsen FA: Fasciotomy in the treatment of the acute compartment syndrome. J Bone Jt Surg (Am) 58:112- 115 1976
- Mubarak SJ Owen CA Hargens AR et al: Acute compartment syndromes: diagnosis and treatment with the aid of the wick catheter. J Bone Jt Surg (Am) 60:1091-1095 1978
- Sirbu AB Murphy MJ White AS: Soft tissue complications of fractures of the leg. Calif Med 60:53-56 1944
- Leach RE Hammond G: The anterior tibial compartment syndrome. Acute and chronic. J Bone Jt Surg (Am) 49:451-462 1967
- Wolfort FG Mogelvang LC Filtzer HS: Anterior tibial compartment syndrome following muscle hernia repair. Arch Surg 106:97-99 1973
- Reneman RS: The anterior and the lateral compartmental syndrome of the leg due to intensive use of muscles. Clin Orthop Relat Res 113:69-80 1975
- Matsen FA Clawson DK: The deep posterior compartmental syndrome of the leg. J Bone Jt Surg (Am) 57:34-39 1975
- Horn JS Sevitt S: Ischaemic necrosis and regeneration of the tibialis anterior muscle after rupture of the popliteal artery. J Bone Jt Surg (Br) 33:348-358 1951
- Bradley EL: The anterior tibial compartment syndrome. Surg Gynecol Obst 136:289-297 1973
- Tilney NL McLamb JR: Leg trauma with posterior tibial artery tear. J Trauma 7:807-810 1967
- McQuillan WM Nolan B: Ischaemia complicating mjury. A report of thirty-seven cases. J Bone Jt Surg (Br) 50:482-492 1968
- Fowler PJ Willis RB: Vascular compartment syndromes. Can J Surg 18:157-161 1975
- Zimmerman JE Afshar F. Firedman W. et al: Posterior compartment syndrome of the thigh with a sciatic palsy. Case report. J Neurosurg 46:369-372 1977
- Thomas HB: Some orthopedic findings in ninety-eight cases of hemophilia. J Bone Jt Surg (Am) 18:140-147 1936
- Bowden REM Gutmann E: The fate of voluntary muscle after vascular injury in man. J Bone Jt Surg (Br) 31:356-368 1949
- Robins RH Murrell JS: Traumatic ischaemia in a haemophiliac. Re port of a case of prolonged haemostasis with cryoprecipitate during decompression and skin graftmg. J Bone Jt Surg (Br) 53:113-117 1971
- Arnold WD Hilgartner MW: Hemophilic arthropathy. J Bone Jt Surg (Am) 59:287-305 1977
- Lancourt JE Gilbert MS Posner MA: Management of bleeding and associated complications of hemophilia in the hand and forearm. J Bone Jt Surg (Am) 59:451-460 1977
- Macon WL Futrell JW: Median-nerve neuropathy after percutaneous F puncture of the brachial artery in patients receiving anticoagulants. N Engl J Med 288:1396 1973
- Neviaser RJ Adams JP May GI: Complications of arterial puncture in anticoagulated patients. J Bone Jt Surg (Am) 58:218-220 1976
- Halpern AA Mochizuki R. Long CE: Compartment syndrome of the forearm following radial-artery puncture in a patient treated with anticoagulants. J Bone Jt Surg (Am) 60:1136-1137 1978
- Reneman RS: The Anterior and the Lateral Compartment Syndrome of the Leg. The Hague Mouton 1968 p 176
- Whitesides TE Hirada H. Morimoto K: The response of skeletal muscle to temporary ischemia: an experimental study. J Bone Jt Surg (Am) 53:1027-1028 1971
- Gitlitz GF: The anterior tibial compartment syndrome. A complication of a femoropopliteal bypass procedure. Vasc Dis 2:122-130 1965
- Coupland GAE: Anterior tibial syndrome following restoration of arterial flow. Aust NZ J Surg 41:338-341 1972
- Lytton B. Blandy JP: Anterior tibial syndrome after embolectomy. Br J Surg 48:346-348 1960
- Ransford AO Provan JL: Anterior tibial compartment syndrome complicating femoral embolectomy. Can J Surg 14:231-234 1971
- Clayton JM Hayes AC Barnes RW: Tissue pressure and perfusion in the compartment syndrome. J Surg Res 22:333-339 1977
- Elliott MJ Glass KD: Anterior tibial compartment syndrome associated with ergotamine ingestion. Clin Orthop Relat Res 118:4447 1976
- Rosengart R. Nelson RJ Emmanoulides GC: Anterior tibial compartment syndrome in a child: an unusual complication of cardiac catheterization. Pediatrics 58:456-458 1976
- LaForce FM: Crush syndrome after ethanol. N Engl J Med 284:1104 1971
- Conner AN: Prolonged external pressure as a cause of ischemic contracture. J Bone Jt Surg (Br) 53:118-122 1971
- Schreiber SN Liebowitz MR Bernstein LH: Limb compression and renal impairment (crush syndrome) following narcotic and sedative overdose. J Bone Jt Surg (Am) 54:1683-1692 1972
- Spinner MA Mache A Silver L et al: Impending ischemic contracture of the hand. Plast Reconstr Surg 50:341-349 1972
- Dolich BH Aiache AE: Drug-induced coma: a cause of crush syndrome and ischemic contracture. J Trauma 13:223-228 1973
- Mubarak SJ Owen CA: Compartmental syndrome and its relation to the crush syndrome: a spectrum of disease. A review of 11 cases of prolonged limb compression. Clin Orthop Relat Res 113:81-89 1975
- Patterson VH Boddie HG: Anterior tibial compartment syndrome associated with alcohol abuse. Br Med J 1:269-270 1977
- Owen CA Mubarak SJ Hargens AR et al: Intramuscular pressure with limb compression. Clarification of the pathogenesis of the druginduced compartment syndrome/crush syndrome. N Engl J Med 300:1169-1172 1979
- Parkes AR: Traumatic ischaemia of peripheral nerves with some observations on Volkmann's ischaemic contracture. Br J Surg 32:403-414 1945
- Ernst CB Kaufer H: Fibulectomy-fasciotomy. An important adjunct in the management of lower extremity arterial trauma. J Trauma 11:365-380 1971v
- Holden CEA: Traumatic tension ischaemia in muscles. Injury 5:223-227 1973
- Horwitz T: Ischemic contracture of the lower extremity. Arch Surg 41:945-959 1940
- Rorabeck CH MacNab I: Anterior tibial-compartment syndrome complicating fractures of the shaft of the tibia. J Bone Jt Surg (Am) 58:549-550 1976
- Onnerfalt R: Fracture of the tibial shaft treated by primary operation and early weight-bearing. Acta Orthop Scand Suppl 171:7-63 1978
- Phalen GD: Ischemic necrosis of the anterior crural muscles. Ann Surg 127:112-120 1948
- Carter AB Richards RL Zachary RB: The anterior tibial syndrome. Lancet II:928-934 1949
- Hughes JR: The anterior tibial syndrome. Lancet II:1150 1949
- Tillotson JF Coventry MB: Spontaneous ischemic necrosis of the anterior tibial muscle: report of case. Proc Staff Meetings Mayo Clin 25:223-227 1950
- Mavor GE: The anterior tibial syndrome. J Bone Jt Surg (Br) 38:513-517 1956
- French EZ Price WH: Anterior tibial pain. Br Med J 2:1290-1296 1962
- Paton DF: The pathogenesis of anterior tibial syndrome. An illustrative case. J Bone Jt Surg (Br) 50:383-385 1968
- Kirby NG: Exercise ischaemia in the fascial compartment of soleus. Report of a case. J Bone Jt Surg (Br) 52:738-745 1970
- Patman RD: Compartmental syndromes in peripheral vascular surgery. Clin Orthop Relat Res 113:103-110 1975
- Garfin S. Mubarak SJ Owen CA: Exertional anterolateral compartmental syndrome. Case report with fascial defect muscle herniation and superficial peroneal-nerve entrapment. J Bone Jt Surg (Am) 59:404-405 1977
- Tompkins DG: Exercise myopathy of the extensor carpi ulnaris muscle. Report of a case. J Bone Jt Surg (Am) 59:407-408 1977
- Mubarak SJ Owen CA Garfin S. et al: Acute exertional superficial posterior compartment syndrome. Am J Sports Med 6:287-290 1978
- Caldwell RK: Ischemic necrosis of the anterior tibial muscle: case report with autopsy findings and review of the literature. Ann Intern Med 46:1191 - 1199 1957
- Manson IW: Post-partum eclampsia complicated by anterior tibial syndrome. Br Med J 2:1117-1118 1964
- Lees AJ: Anterior tibial compartment syndrome following prolonged tetany. J Neurol Neurosurg Psychiatry 39:406-408 1976
- Morgan NR Waugh TR Boback MD: Volkmann's ischemic contracture after intra-arterial injection of secobarbital. JAMA (J Am Med Assoc) 212:476-478 1970
- Hawkins LG Lischer CG Sweeney M: The main line accidental intra-arterial drug injection. Clin Orthop Relat Res 94:268-274 1973
- Kaufer H. Spengler DM Noyes FR et al: Orthopedic implications of the drug subculture. J Trauma 14:853-867 1974
- Schrock RD: Peroneal nerve palsy following derotation osteotomies for tibial torsion. Clin Orthop Relat Res 62:172- 177 1969
- Matsen FA Staheli LT: Neurovascular complications following tibial osteotomy in children. A case report. Clin Orthop Relat Res 110:210-214 1975
- Wiggins HE: The anterior tibial compartment syndrome-a complication of the Hauser procedure. Clin Orthop Relat Res 113:90-94 1975
- Wall JJ: Compartment syndrome as a complication of the Hauser procedure. J Bone Jt Surg (Am) 61:185-191 1979
- Glass TG: Cortisone and immediate fasciotomy in the treatment of severe rattlesnake bite. Tex Med 65:40-47 1969
- Cywes S. Louw JH: Phlegmasia cerulea dolens: successful treatment by relievmg fasciotomy. Surgery 51:169-176 1962
- Weitz EM Carson G: The anterior tibial compartment syndrome in a twenty month old infant. A complication of the use of a bow leg brace. Bull Hosp Jt Dis 30:16-20 1969
- Dennis C: Disaster following femoral vein ligation for thrombophlebitis; relief by fasciotomy; clinical case of renal impairment following crush injury. Surgery 17:264-269 1945
- Sweeney HE O'Brien GF: Bilateral anterior tibial syndrome in association with the nephrotic syndrome: report of a case. Arch Intern Med 116:487-490 1965
- Maor P. Levy M Lotem M et al: Iatrogenic Volkmann's ischemia-a result of pressure-transfusion. Int Surg 57:415-416 1972
- Halpern AA Nagel DA: Bilateral compartment syndrome associated with androgen therapy. A case report. Clin Orthop Relat Res 128:243-246 1977
- Scott NW Jacobs B. Lockshin MD: Posterior compartment syndrome resulting from a dissecting popliteal cyst. Clin Orthop Relat Res 122:189-192 1977
- Ashton H: The effect of increased tissue pressure on blood flow. Clin Orthop Relat Res 113:15-26 1975
- Fuhrman FA Crismon JM: Early changes in distribution of sodium potassium and water in rabbit muscles following release of tourniquets. Am J Physiol 166:424-432 1951
6. Anatomical Locations
- Mubarak SJ Owen CA Hargens AR et al: Acute compartment syndrome: diagnosis and treatment with the aid of the wick catheter. J Bone Jt Surg (Am) 60:1091-1095 1978
- Eaton RG Green WT: Volkmann's ischemia. A volar compartment syndrome of the forearm. Clin Orthop Relat Res 113:58-64 1975
- McQuillan WM Nolan B: Ischaemia complicating injury. A report of thirty-seven cases. J Bone Jt Surg (Br) 50:482-492 1968
- Gelberman RH Zakaib GS Mubarak SJ et al: Decompression of forearm compartment syndromes. Clin Orthop Relat Res 134:225-229 1978
- Patman RD Thompson JE: Fasciotomy in peripheral vascular surgery. Arch Surg 101:663-670 1970
- Tompkins DG: Exercise myopathy of the extensor carpi ulnaris muscle. Report of a case. J Bone Jt Surg (Am) 59:407-408 1977
- Green WT Mital MA: Congenital radio-ulnar synostosis: surgical treatment. J Bone Jt Surg (Am) 61:738-743 1979
- Spinner MA Mache A Silver L et al: Impending ischemic contracture of the hand. Plast Reconstr Surg 50:341-349 1972
- Reid RL Travis RT: Acute necrosis of the second interosseous compartment of the hand. J Bone Jt Surg (Am) 55:1095-1097 1973
- Salisbury RE McKeel DW Mason AD: Ischemic necrosis of the intrinsic muscles of the hand after thermal injuries. J Bone Jt Surg (Am) 56:1701-1707 1974
- Kaufman G. Choi B: Ischemic necrosis of muscles of the buttock. A case report. J Bone Jt Surg (Aml 54:1079-1082 1972
- Klock JC Sexton MJ: Rhabdomyolysis and acute myoglobinuric renal failure following heroin use. Calif Med 119:5-8 1973
- Owen CA Woody PR Mubarak SJ et al: Gluteal compartment syndromes: a report of three cases and management utilizing the wick catheter. Clin Orthop Relat Res 132:57-60 1978
- Tilney NL McLamb JR: Leg trauma with posterior tibial artery tear. J Trauma 7:807-810 1967
- Reneman RS: The Anterior and the Lateral Compartment Syndrome of the Leg. The Hague Mouton 1968 p 176
- Shrock RD: Peroneal nerve palsy following derotation osteotomies for tibial torsion. Clin Orthop Relat Res 62:172-177 1969
- Bradley EL: The anterior tibial compartment syndrome. Surg Gynecol Obst 136:289-297 1973
- Wiggins HE: The anterior compartment syndrome-a complication of the Hauser procedure. J Bone Jt Surg (Am) 55-A:1306 1973
- Rorabeck CH MacNab I: Anterior tibial-compartment syndrome complicating fractures of the shaft of the tibia. J Bone Jt Surg (Am) 58:549-550 1976
- Garfin S. Mubarak SJ Owen CA: Exertional anterolateralcompartment syndrome. Case report with fascial defect muscle herniation and superficial peroneal-nerve entrapment. J Bone Jt Surg (Am) 59:404405 1977
- Reszel PA Janes JM Spittell JA: Ischemic necrosis of the peroneal musculature. A lateral compartment syndrome: report of a case. Mayo Clin Proc 38: 130-136 1963
- Davies JAK: Peroneal compartment syndrome secondary to rupture of the peroneus longus. J Bone Jt Surg (Am) 61:783-784 1979
- Kirby NG: Exercise ischaemia in the fascial compartment of soleus. Report of a case. J Bone Jt Surg (Br) 52:738-740 1970
- Lowenberg EL: Acute ischemic infarction of the gastrocnemius muscle simulating deep vein phlebitis. J Cardiovasc Surg 6:104-110 1965
- Mubarak SJ Owen CA Garfin S. et al: Acute exertional superficial posterior compartment syndrome. Am J Sports Med 6:287-290 1978
- Matsen FA Clawson DK: The deep posterior compartmental syndrome of the leg. J Bone Jt Surg (Am) 57:34-39 1975
- Whitesides TE Harada H. Morimoto K: Compartment syndromes and the role of fasciotomy its parameters and techniques in Instructional Course Lectures The American Academy of Orthopedic Surgeons vol 26. St Louis Mosby 1977 p 179
- Sheridan GW Matsen FA: Fasciotomy in the treatment of the acute compartment syndrome. J Bone Jt Surg (Am) 58:112-115 1976
7. Diagnosis
- Dahn I Lassen NA Westling H: Blood flow in human muscles during external pressure or venous stasis. Clin Sci 32:467-473 1967
- Clayton JM Hayes AC Barnes RW: Tissue pressure and perfusion in the compartment syndrome. J Surg Res 22:333-339 1977
- Sheridan GW Matsen FA Krugmire RB: Further investigations on the pathophysiology of the compartmental syndrome. Clin Orthop Relat Res 123:266-270 1977
- Rorabeck CH Clarke KM: The pathophysiology of anterior tibial compartment syndrome: an experimental investigation. J Trauma 18:299-304 1978
- Matsen FA Krugmire RB King RV: Increased tissue pressure and its effects on muscle oxygenation in level and elevated human limbs. Nicholas Andry Award. Clin Orthop Relat Res 144:311-320 1979
- Matsen FA King RV Krugnure RB et al: Physiological effects of increased tissue pressure. Int Orthop SICOT 3:237-244 1979
- Matsen FA Mayo KA Krugmire RB et al: A model compartmental syndrome in man with particular reference to the quantification of nerve function. J Bone Jt Surg (Am) 59:648-653 1977
- Matsen FA Winquist RA Krugmire RB: Diagnosis and management of compartmental syndromes. J Bone Jt Surg (Am) 62:286-291 1980
- Dahlback L-O: Effects of temporary tourniquet ischemia on striated muscle fibers and motor end-plates. Scand J Plast Reconstr Surg Suppl 7 7-91 1970
- Lundborg G: Ischemic nerve injury. Scand J Plast Reconstr Surg Suppl 6 3-113 1970
- Sheridan GW Matsen FA: Fasciotomy in the treatment of the acute compartment syndrome. J Bone Jt Surg (Am) 58:112-115 1976
- Matsen FA Clawson DK: The deep posterior compartmental syndrome of the leg. J Bone Jt Surg (Am) 57:34-39 1975
- Veith RG: Unpublished data
- Matsen FA Staheli LT: Neurovascular complications following tibial osteotomy in children: a case report. Clin Orthop Relat Res 110:210-214 1975
8. Treatment
- Holden CEA: Traumatic tension ischaemia in muscles. Injury 5:223-227 1973
- Eaton RG Green WT: Volkmann's ischemia. A volar compartment syndrome of the forearm. Clin Orthop Relat Res 113:58-64 1975
- Holden CEA: Compartmental syndromes following trauma. Clin Orthop Relat Res 113:95-102 1975
- Matsen FA: Compartmental syndromes. A unified concept. Clin Orthop Relat Res 113:8-14 1975
- Matsen FA Clawson DK: The deep posterior compartmental syndrome of the leg. J Bone Jt Surg (Am) 57:34-39 1975
- Sheridan GW Matsen FA: Fasciotomy in the treatment of the acute compartment syndrome. J Bone Jt Surg (Am) 58:112-115 1976
- Vracko R. Benditt EP: Basal lamina: the scaffold for orderly cell replacement. Observation on regeneration of injured skeletal muscle fibers and capillaries. J Cell Biol 55:406-419 1972
- Sanderson RA Foley RK McIvor GWD et al: Histological response of skeletal muscle to ischemia. Clin Orthop Relat Res 113:27-35 1975
- Whitesides TE Harada H. Morimoto K: Compartmental syndromes and the role of fasciotomy its parameters and techniques in Instructional Course Lectures The American Academy of Orthopedic Surgeons vol 26. St Louis Mosby 1977 p 179
- Gaspard DJ Kohl RD: Compartmental syndromes in which the skin is the limiting boundary. Clin Orthop Relat Res 113:65-68 1975
- Masten FA Winquist RA Krugmire RB: Diagnosis and management of compartmental syndromes. J Bone Jt Surg (Am) 62:286-291 1980
- Patman RD: Compartmental syndromes in peripheral vascular surgery. Clin Orthop Relat Res 113:103-110 1975
9. Sequelae
- Sheridan GW Matsen FA: Fasciotomy in the treatment of the acute compartment syndrome. J Bone Jt Surg (Am) 58:112-115 1976
- Albo D Cheung L Ruth L et al: Effect of intra-arterial injections of barbiturates. Am J Surg 120:676-678 1970
- Gaspar MR Hare RR: Gangrene due to intra-arterial injection of drugs by drug addicts. Surgery 72:573-577 1972
- Kaufer H. Spengler DM Noyes FR et al: Orthopedic implications of the drug subculture. J Trauma 14:853-867 1974
- Montagnani CA Simeone FA: Observations on the liberation and elimination of myohemoglobin and of hemoglobin after release of muscleischemia. Surgery34:169-185 1953
- Schreiber SN Liebowitz MR Bernstein LH et al: Limb compression and renal impairment (crush syndrome) complicating narcotic overdose. N Engl J Med 284:368-369 1971
- Spinner MA Mache A Silver L et al: Impending ischemic contracture of the hand. Plast Reconstr Surg 50:341-349 1972
- Klock JC Sexton MJ: Rhabdomyolysis and acute myoglobinuric renal failure following heroin use. Calif Med 119:5-8 1973
- Owen CA Mubarak SJ Hargens AR et al: Intramuscular pressure with limb compression. Clarification of the pathogenesis of the druginduced compartment syndrome/crush syndrome. N Engl J Med 300:1169-1172 1979
- Mubarak SJ Owen CA: Compartmental syndrome and its relation to the crush syndrome: a spectrum of disease. A review of 11 cases of prolonged limb compression. Clin Orthop Relat Res 113:81-89 1975
- Tsuge K: Treatment of established Volkmann's contracture. J Bone Jt Surg (Am) 57:925-929 1975
- Miller DS Markin L Grossman E: Ischemic fibrosis of the lower extremity in children. Am J Surg 84:317-321 1952
- Karlstrom G. Olerud S: Cavus deformity of the foot after fracture of the tibial shaft. J Bone Jt Surg (Am) 57:893-900 1975
- Matsen FA Clawson DK: The deep posterior compartmental syndrome of the leg. J Bone Jt Surg (Am) 57:34-39 1975
- Clark MW D'Ambrosia RD Roberts JM: Equinus contracture following Bryant's traction. Orthopedics 1:311-312 1978
- Coupland GAE: Anterior tibial syndrome following restoration of arterial flow. Aust NZ J Surg 41:338-341 1972
10. Recurrent compartmental syndromes
- Mavor GE: The anterior tibial syndrome. J Bone Jt Surg (Br) 38:513-517 1956
- Kunkel MG Lynn RB: The anterior tibial compartment syndrome. Can J Surg 1:212-217 1958
- French EZ Price WH: Anterior tibial pain. Br Med J 2:1290-1296 1962
- Leach RE Hammond G: The anterior tibial compartment syndrome. Acute and chronic. J Bone Jt Surg (Am) 49:451-462 1967
- Reneman RS: The Anterior and the Lateral Compartment Syndrome of the Leg. The Hague Mouton 1968 p 176
- Reneman RS: The anterior and the lateral compartmental syndrome of the leg due to intensive use of muscles. Clin Orthop Relat Res 113:69-80 1975
- Barcroft J. Kato T: Effects of functional activity in striated muscle and the submaxillary gland. Philos Trans R Soc Lond B Biol Sci 207: 149- 182 1915/1916
- Kjellmer I: An indirect method for estimating tissue pressure with special reference to tissue pressure in muscle during exercise. Acta Physiol Scand 62:31-40 1964
- Arai M Endoh H: Blood flow through human skeletal muscle during and after contraction. Tohoku J Exp Med 114:379-384 1974
- Mubarak SJ Hargens AR Owen CA et al: The wick catheter tech nique for measurement of intramuscular pressure. A new research and clinical tool. J Bone Jt Surg (Am) 58:1016-1021 1976
- Garfin S. Mubarak SJ Owen CA: Exertional anterolateralcompartment syndrome. Case report with fascial defect muscle herniation and superficial peroneal-nerve entrapment. J Bone Jt Surg IAm) 59:404-405 1977
- Puranen J: The medial tibial syndrome. Exercise ischaemia in the medial fascial compartment of the leg. J Bone Jt Surg (Br) 56:712-715 1974
Fronticepiece
". . . I'll just see what this bottle does. I hope it'll make me grow large again for really I'm quite tired of being such a tiny little thing."
It did so indeed and much sooner than she had expected: before she had drunk half the bottle she found her head pressing against the ceiling and had to stoop to save her neck from being broken. She hastily put down the bottle saying to herself "that's quite enough-I hope I shan't grow anymore-As it is I can't get out at the door-I do wish I hadn't drunk quite so much!" Alas! It was too late to wish that! She went on growing and growing and very soon had to kneel down on the floor: in another minute there was not even room for this and she tried the effect of lying down with one elbow against the door and the other arm curled round her head. Still she went on growing and as a last resource she put one arm out of the window and one foot up the chimney and said to herself "Now I can do no more whatever happens. What will become of me?"
(Reprinted with permission from The Annotated Alice: Alice's Adventures in Wonderland Through the Looking Glass by Lewis Carroll illustrated by John Tenniel with an Introduction and Notes by Martin Gardner. Published by Clarkson N. Potter New York t960 p. 57.)
Library of Congress Cataloging in Publication Data
Matsen Frederick A. Compartmental syndromes.
Includes bibliographical references and index.
- Compartment syndrome. I. Title. RC951.M33 616.1'31 80-14840 ISBN 0-8089-1260-7
- by Grune & Stratton Inc.
All rights reserved. No part of this publication may be reproduced or transmitted in any form or by any means electronic or mechanical including photocopy recording or any information storage and retrieval system without permission in writing from the publisher.
Grune & Stratton Inc. 111 Fifth Avenue New York New York 10003
Distributed in the United Kingdom by Academic Press Inc. (London) Ltd. 24/28 Oval Road London NW 1
Library of Congress Catalog Number 80-14840 International Standard Book Number 0-8089-1260-7 Printed in the United States of America
To my wife Anne my children Susanna Erick and Laura my parents Al and Ceil and my late friend Dick Krugmire
Acknowledgments
It is always a pleasure to thank associates for their invaluable help. I must first acknowledge Dr. D. Kay Clawson who stimulated my interest in compartmental syndromes while I was one of his residents and encouraged me to set up the Limb Viability Laboratory and Dr. Victor H. Frankel under whose chairmanship the laboratory has grown and prospered.
One of the mainstays of the laboratory since its inception was the late Richard B. Krugmire Jr. who graciously volunteered hundreds of after-work and weekend hours for these investigations. His friendship and encouragement saw me through many challenging times. Dick's many contributions to our investigations are evidenced in this book; his memory lives in its pages.
It can easily be seen that Dr. Geoffrey Sheridan has contributed much to the fund of knowledge on compartmental syndromes and to the laboratory. His work on animal model systems and clinical observations on the timing of surgical decompression form much of the basis for our current management of patients at risk for compartmental syndromes. As a medical student Keith Mayo gave us a great deal of help in the development of the infusion system for tissue pressure measurement. His legs also joined mine Sheridan's and Krugmire's in providing "normal volunteers for many of the human studies.
Sarah Sato has done triple duty serving as my clinical secretary as secretary for the Limb Viability Laboratory and as an encouraging friend. Without her help much of this work could not have been accomplished. Racheal King is the Limb Viability Laboratory's research technologist par excellence; among her contributions I must acknowledge that she is the one who can keep the mass spectrometer working. Laurie Glass has edited this book and many of the papers on which it is based. She demonstrated a wonderful ability to comprehend this information and to assure its lucid presentation. Her many hours of constructive criticism have been invaluable. Dale Leuthold has produced most of the illustrations for this book. Her quick grasp of the concepts to be illustrated and her precise artwork have greatly facilitated the presentation of this material. There are many other friends both doctor and patient who have greatly enhanced my understanding of this subject. With an apology for not citing all of their names I would like to thank them for their help.
Finally I graciously acknowledge the support of the National Institutes of Health Grants No. ROI AM18642-01 -2 -03; the Orthopedic Research Education Foundation Grants No. 244 and 266; and the Prosthetic Research Study under the directorship of Dr. Ernest M. Burgess. This support has been essential to our progress.
Foreword
The readers of this text are indebted to Dr. Matsen for this beautiful summation work on compartmental syndromes. While the author states that this text is "not a chronological review of the history of compartmental syndromes it certainly beautifully and completely covers the subject. Every physician, especially the orthopedic surgeon who deals with so much trauma, must be totally familiar not only with the concept of compartmental syndromes but with the diagnostic criteria and the proper treatment. Certainly the majority of the data in this book concerns acute compartmental syndromes, but a very important aspect, the recurrent compartmental syndrome, is nicely covered by Dr. Veith.
Matsen points out that because of inadequate literature indexing, it is difficult to locate relevant articles on this subject. This text, because of its extensive bibliography on each chapter, will become the classic reference on the subject.
I have participated with Dr. Matsen in teaching conferences over the past three years and know him to be a dedicated and careful contributor. His work in the Limb Viability Laboratory at the University of Washington has led to this creative text which will be of value for many years to come. He and his colleagues are to be congratulated for this significant contribution.
Charles A. Rockwood, Jr., M.D. Professor and Chairman Division of Orthopedics University of Texas Medical School at San Antonio
Preface
Writing this book provided an opportunity to relate what seems to be important about compartmental syndromes. It is not a review of the literature for that would be more confusing than informative. It is not a chronological review of the history of compartmental syndromes because history took many wrong turns that need not be recounted. Rather this is a practical book designed to provide the clinician the physiologist the resident physician and the student with a detailed view of this most interesting and important condition.
Most of the investigative data presented in this volume originated in the Limb Viability Laboratory at the University of Washington. Similarly many of the clinical observations have been made in the University of Washington's affiliated hospitals particularly the University Hospital Harborview Medical Center and Children's Orthopedic Hospital. This book represents a synthesis of this laboratory and clinical experience and other available data in a form that I hope will be useful to clinician and scientist alike.
The book begins with a short section setting forth the problems encountered in dealing with compartmental syndromes. Next Chapter 1 gives a definition of the compartmental syndrome that I hope will obviate much of the confusion that surrounds this condition. Chapter 2 defines "tissue pressure which may be another confusing concept. Different techniques for the measurement of tissue pressure are also described in this chapter. Included is a practical guide to the use of the infusion technique, which I find most useful for continuously monitoring patients at risk for compartmental syndromes. Chapter 3 is devoted to the pathophysiology of increased tissue pressure. Of particular concern is the mechanism by which increased tissue pressure compromises local circulation. In Chapter 4 the factors affecting the tolerance of tissue for increased tissue pressure are discussed. These factors determine the susceptibility to a compartmental syndrome and suggest some methods by which this susceptibility may be lessened. Chapters 5 and 6 present the common etiologies and anatomical locations of compartmental syndromes. Of primary importance is the observation that increased tissue pressure from any cause in any location may potentially produce a compartmental syndrome.
Chapter 7 reviews the diagnosis of compartmental syndromes, emphasizing the importance of clinical symptoms and signs. Adjunctive diagnostic techniques of tissue pressure measurement and direct nerve stimulation are discussed as well. Chapter 8 presents the treatment of compartmental syndromes and deals not only with techniques of surgical decompression but also with management of associated fractures and wound closure following surgical decompression. Chapter 9 presents the sequelae of compartmental syndromes such as contractures, paralysis, infection, and myoglobinuric renal failure.
Our experience has yielded some preventive measures that may reduce the incidence of compartmental syndromes. These are presented in Chapter 10. Chapter 11 synthesizes the important practical points in a clinical approach to patients at risk for acute compartmental syndromes. This approach is designed to minimize the frequency of compartmental syndromes and to assist in their prompt diagnosis and effective treatment.
Chapters 7 through 11 are concerned primarily with acute compartmental syndromes. In Chapter 12 Dr. Robert G. Veith discusses recurrent compartmental syndromes due to intensive use of muscles. These conditions are becoming more important with the increasing interest in endurance sports such as long-distance running, walking, and canoeing. Chapter 13 enables readers to test their knowledge on some challenging diagnostic and treatment problems.
If you are a clinician, I hope this book will help you better understand the compartmental syndrome so that you may prevent your patients from falling victim to its sequelae. If you are a scientist, I hope this book will stimulate your interest in this unique example of local circulatory failure, which has too long been ignored by those who potentially hold the keys to understanding it. I am most interested in your views on the information presented here and would gladly welcome any correspondence from you.
Frederick A. Matsen III, M.D.

