Edited By Frederick A. Matsen III, M.D., Professor, UW Orthopaedics & Sports Medicine

Last updated: October 19, 2011
For the most up to date information on shoulder arthritis, follow our blog:
http://shoulderarthritis.blogspot.com/
Contact
If you have questions regarding the surgical treatment of arthritis, feel free to email Frederick A. Matsen III, M.D. at matsen@uw.edu.
Overview
Motion and function of joints
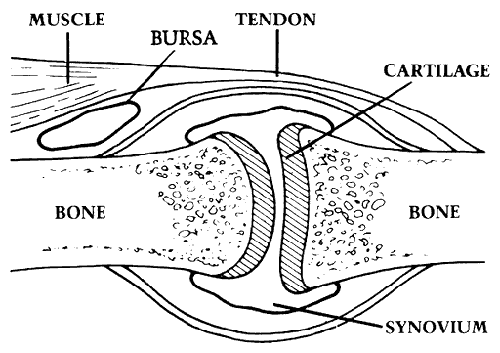
Human bones join with each other in a variety of ways to serve the functional requirements of the musculoskeletal system. Foremost among these needs is that of purposeful motion. The activities of the human body depend on effective interaction between normal joints and the neuromuscular units that drive them. The same elements also interact reflexively to distribute mechanical stresses among the tissues of the joint. Muscles, tendons, ligaments, cartilage and bone all do their share to ensure smooth function (see figure 1). In this role, the supporting elements both unite the abutting bones and position the joints in the optimal relationship for low-friction load-bearing. Two important characteristics of normal joint function are stability and lubrication.
Cartilage
The cartilage covering our joint surfaces is called "articular cartilage." Normally, it is a smooth well-lubricated surface that offers less frictional resistance than that of an ice skate gliding on ice.
Normal cartilage is very durable and somewhat elastic, providing a shock absorber for our joints. Articular cartilage does not have a blood supply. Rather it gets it oxygen and nutrients from the surrounding joint fluid. When a joint is loaded, the pressure squeezes fluid including waste products out of the cartilage, and when the pressure is relieved, the fluid seeps back in together with oxygen and nutrients. Thus, the health of cartilage depends on it being used. Unfortunately, once it is injured, cartilage has a limited ability to repair itself.
Damaged or abnormal cartilage loses it resistance to wear. The two joint surfaces grate one on the other and shed particles of cartilage which further contribute to joint surface wear. As the joint mechanics deteriorate, the rate of wear increases. The process may continue until most of the joint cartilage is gone. Bone spurs seem to be the body's attempt to provide more joint surface however because these bone spurs are not covered by normal cartilage, the affect is not helpful. The wearing of cartilage may produce deformities such as bowed legs or stiff spines. Loose pieces of bone and cartilage may break off and cause joints to "lock".
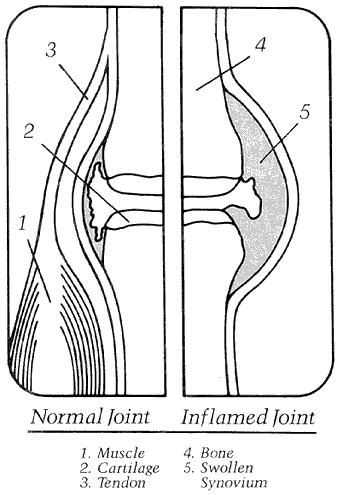
Joint inflammation
versus inflamed joint
What is inflammation?
Many types of arthritis are characterized by inflammation. Inflammation is a part of the body's healing response characterized by swelling redness and warmth (see figure 2). This response is stimulated by injury, infection, surgery and allergic reactions.
Normally, this inflammatory response removes unhealthy and foreign material from the area. It also begins the repair process in which new blood vessels and tissue-rebuilding cells (fibroblasts) come to the injury site. The body's immune system can be viewed much like a demolition company that tears down old buildings so that new ones can be built.
Inflammation in joints
In some types of arthritis, such as rheumatoid arthritis, the body's immune system gets confused and acts as if joint cartilage doesn't belong there. The signs of joint inflammation are typical findings.
This is called an autoimmune response. In other words, the demolition company starts in on an essential building that cannot be rebuilt. Sometimes the inflammation does not stop until the cartilage has been removed from the joint.
Joint stability
Factors of stability
A number of factors interact to confer stability while permitting motion in active human joints. First among these is the shape of the component parts. In the hips, for example, weight bearing drives the femoral head into a relatively deep socket, the acetabulum. The articular members are configured and positioned so that normal loading enhances the closeness of their fit.
Ligaments provide a second major stabilizing influence as they guide and align normal joints through their range of motion. An excellent example is the collateral and cruciate ligaments of the knee. These strong relatively inelastic structures limit articular motion to flexion and extension.
Within the axes of motion, however, more flexible constraints are required. This need is met by muscles and tendons. Muscular stabilization is perhaps most obvious in the shoulder, which is the quintessential polyaxial joint. The rotator cuff muscles approximate and stabilize the articular surfaces of the shoulder as larger muscles with better leverage provide the power for effective shoulder motion.
Movies
Synovial fluid
Synovial fluid contributes significant stabilizing effects as an adhesive seal that freely permits sliding motion between cartilaginous surfaces while effectively resisting distracting forces. This property is most easily demonstrated in small articulations, such as the metacarpophalangeal joints. The common phenomenon of "knuckle cracking" reflects the fracture of this adhesive bond. Secondary cavitation within the joint space causes a radiologically obvious bubble of gas that requires up to 30 minutes to dissolve before the bond can be reestablished and the joint can be "cracked" again. This adhesive property depends on the normally thin film of synovial fluid between all intraarticular structures. When this film enlarges as a pathologic effusion, the stabilizing properties are lost.
In normal human joints, a thin film of synovial fluid covers the surfaces of synovium and cartilage within the joint space. The volume of this fluid increases when disease is present to provide an effusion that is clinically apparent and may be easily aspirated for study. For this reason, most knowledge of human synovial fluid comes from patients with joint disease. Because of the clinical frequency, volume and accessibility of knee effusions, our knowledge is largely limited to findings in that joint.
In the synovium, as in all tissues, essential nutrients are delivered and metabolic by-products are cleared by the bloodstream perfusing the local vasculature. Synovial microvessels contain fenestrations that facilitate diffusion-based exchange between plasma and the surrounding interstitium. Free diffusion provides full equilibration of small solutes between plasma and the immediate interstitial space. Further diffusion extends this equilibration process to include all other intracapsular spaces including the synovial fluid and the interstitial fluid of cartilage. Synovial plasma flow and the narrow diffusion path between synovial lining cells provide the principal limitations on exchange rates between plasma and synovial fluid.
This process is clinically relevant to the transport of therapeutic agents in inflamed synovial joints. Many investigators have made serial observations of drug concentrations in plasma and synovial fluid after oral or intravenous administration. Predictably plasma levels exceed those in synovial fluid during the early phases of absorption and distribution. This gradient reverses during the subsequent period of elimination when intrasynovial levels exceed those of plasma. These patterns reflect passive diffusion alone and no therapeutic agent is known to be transported into or selectively retained within the joint space.
Metabolic evidence of ischemia provides a second instance when the delivery and removal of small solutes becomes clinically relevant. In normal joints and in most pathologic effusions, essentially full equilibration exists between plasma and synovial fluid. The gradients that drive net delivery of nutrients (glucose and oxygen) or removal of wastes (lactate and carbon dioxide) are too small to be detected. In some cases, however, the synovial microvascular supply is unable to meet local metabolic demand and significant gradients develop. In these joints, the synovial fluid reveals a low oxygen pressure (PO2) low glucose, low pH, high lactate and high carbon dioxide pressure (PCO2). Such fluids are found regularly in septic arthritis, often in rheumatoid disease, and infrequently in other kinds of synovitis. Such findings presumably reflect both the increased metabolic demand of hyperplastic tissue and impaired microvascular supply.
Consistent with this interpretation is the finding that ischemic rheumatoid joints are colder than joints containing synovial fluid in full equilibration with plasma. Like other peripheral tissues, joints normally have temperatures lower than that of the body's core. The knee, for instance, has a normal intraarticular temperature of 32°C. With acute local inflammation, articular blood flow increases and the temperature approaches 37°C. As rheumatoid synovitis persists, however, microcirculatory compromise may cause the temperature to fall as the tissues become ischemic.
The clinical implications of local ischemia remain under investigation. Decreased synovial fluid pH, for instance, was found to correlate strongly with radiographic evidence of joint damage in rheumatoid knees. Other work has shown that either joint flexion or quadriceps contraction may increase intrasynovial pressure, and thereby exert a tamponade effect on the synovial vasculature. This finding suggests that normal use of swollen joints may create a cycle of ischemia and reperfusion that leads to tissue damage by toxic oxygen radicals.
Normal articular cartilage has no microvascular supply of its own and therefore is at risk in ischemic joints. In this tissue, the normal process of diffusion is supplemented by the convection induced by cyclic compression and release during joint usage. In immature joints, the same pumping process promotes exchange of small molecules with the interstitial fluid of underlying trabecular bone. In adults however this potential route of supply is considered unlikely and all exchange of solutes may occur through synovial fluid. This means that normal chondrocytes are farther from their supporting microvasculature than are any other cells in the body. The vulnerability of this extended supply line is clearly shown in synovial ischemia.
The normal proteins of plasma also enter synovial fluid by passive diffusion. In contrast to small molecules, however, protein concentrations remain substantially less in synovial fluid than in plasma. In aspirates from normal knees, the total protein was only 1.3 g/dL, a value roughly 20% of that in normal plasma. Moreover, the distribution of intrasynovial proteins differs from that found in plasma. Large proteins such as IgM and cr2-macroglobulin are underrepresented whereas smaller proteins are present in relatively higher concentrations. The mechanism determining this pattern is reasonably well understood. The microvascular endothelium provides the major barrier limiting the escape of plasma proteins into the surrounding synovial interstitium. The protein path across the endothelium is not yet clear; conflicting experimental evidence supports the fenestrae, intercellular junctions, and cytoplasmic vesicles as the predominant sites of plasma protein escape. What does seem clear is that the process follows diffusion kinetics. This means that smaller proteins which have fast diffusion coefficients will enter the joint space at rates proportionately faster than those of large proteins with relatively slow diffusion coefficients.
In contrast, proteins leave synovial fluid through Iymphatic vessels, a process that is not size-selective. Protein clearance may vary with joint disease. In particular, joints affected by rheumatoid arthritis (RA) experience significantly more rapid removal of proteins than do those of patients with osteoarthritis. Thus, in all joints, there is a continuing, passive transport of plasma proteins involving synovial delivery in the microvasculature, diffusion across the endothelium and ultimate Iymphatic return to plasma.
The intrasynovial concentration of any protein represents the net contributions of plasma concentration, synovial blood flow, microvascular permeability, and Iymphatic removal. Specific proteins may be produced or consumed within the joint space. Thus, lubricin is normally synthesized within synovial cells and released into synovial fluid where it facilitates boundary layer lubrication of the cartilage-on-cartilage bearing. In disease, additional proteins may be synthesized such as IgG rheumatoid factor in RA or released by inflammatory cells, such as Iysosomal enzymes. In contrast, intraarticular proteins may be depleted by local consumption, as are complement components in rheumatoid disease.
Synovial fluid protein concentrations vary little between highly inflamed rheumatoid joints and modestly involved osteoarthritic articulations. Microvascular permeability to protein, however, is more than twice as great in RA as in osteoarthritis. This marked difference in permeability leads to only a minimal increase in protein concentration because the enhanced ingress of proteins is largely offset by a comparable rise in Iymphatic egress. These findings illustrate that synovial microvascular permeability cannot be evaluated from protein concentrations unless the kinetics of delivery or removal are concurrently assessed.
Intraarticular pressure
Intraarticular pressure is about -4 mmHg in the resting normal knee and this pressure falls farther when the quadriceps muscle contracts. The difference between atmospheric pressure on overlying tissues and subatmospheric values within the joint helps to hold the joint members together and thus provides a stabilizing force. In a pathologic effusion, however, the resting pressure is above that of the atmosphere and it rises farther when surrounding muscles contract. Thus, reversal of the normal pressure gradient is an additional destabilizing factor in joints with effusions.
Joint lubrication
How are joints lubricated?
Synovial joints act as mechanical bearings that facilitate the work of the musculoskeletal machine. As such, normal joints are remarkably effective with coefficients of friction lower than those obtainable with manufactured journal bearings. Furthermore, the constant process of renewal and restoration ensures that living articular tissues have a durability far superior to that of any artificial bearing. No artificial joint can equal the performance of a normal human joint.
The mechanics of joint lubrication have provided a focus of investigation beginning with the unique structure of the bearing surface. Articular cartilage is elastic, fluid-filled and backed by a relatively impervious layer of calcified cartilage and bone. This means that load-induced compression of cartilage will force interstitial fluid to flow laterally within the tissue and to surface through adjacent cartilage. As that area in turn becomes load bearing, it is partially protected by the newly expressed fluid above it. This is a special form of hydrodynamic lubrication so-called because the dynamic motion of the bearing areas produces an aqueous layer that separates and protects the contact points.
Boundary layer lubrication is the second major low-friction characteristic of normal joints. Here the critical factor is proposed to be a small glycoprotein called lubricin. The lubricating properties of this synovium-derived molecule are highly specific and depend on its ability to bind to articular cartilage where it retains a protective layer of water molecules. Lubricin is not effective in artificial systems and thus does not lubricate artificial joints.
Other lubricating mechanisms have been proposed; some remain under investigation. Interestingly, hyaluronic acid, the molecule that makes synovial fluid viscous (synovia means "like egg white") has largely been excluded as a lubricant of the cartilage-on-cartilage bearing. Instead, hyaluronate lubricates a quite different site of surface contact-that of synovium on cartilage. The well-vascularized, well-innervated synovium must alternately contract and then expand to cover non-loaded cartilage surfaces as each joint moves through its normal range of motion. This process must proceed freely. Were synovial tissue to be pinched, there would be immediate pain, intraarticular bleeding and inevitable functional compromise. The rarity of these problems testifies to the effectiveness of hyaluronate-mediated synovial lubrication.
Joint popping and cracking
Why do joints make popping or cracking noises?
Joints can make different noises--some are serious and some are not.
Some people learn how to "pop their knuckles." By pushing or pulling a joint in a certain way, an air bubble can suddenly appear in the joint with a "pop." Once the bubble is there, the joint cannot be popped again until the air has been reabsorbed.
Some joints crack as the ligaments and tendons that pass over them slide past bumps on the bones. Individuals who "crack their neck" make noise in this way.
Other joints lock up intermittently--often with a loud pop--because something gets caught in between the joint surfaces. A torn cartilage in the knee or a loose piece of bone or cartilage in the joint can do this. Once a joint is stuck in this way, it may need to be wiggled around to unlock it. This may also cause a pop.
Finally, joints that are arthritic may crack and grind. These noises usually occur each time the joint is moved. This noise is due to the roughness of the joint surface due to loss of the smooth cartilage.
Credits
Some of this material adapted from a chapter in the "Primer on the Rheumatic Diseases" originally prepared by Peter A. Simkin, M.D.
Some of this material adapted from information originally prepared for the Arthritis Foundation.
This material is protected by copyright.